Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| Light-enhanced dark respiration | LEDR | Light-enhanced dark respiration LEDR is a sharp (negative) maximum of dark respiration in plants in response to illumination, measured immediately after switching off the light. LEDR is supported by respiratory substrates produced during photosynthesis and closely reflects light-enhanced photorespiration (Xue et al 1996). Based on this assumption, the total photosynthetic oxygen flux TP is calculated as the sum of the measured net photosynthetic oxygen flux NP plus the absolute value of LEDR. |
| Limiting pO2 | plim | In the transition from aerobic to anaerobic metabolism, there is a limiting pO2, plim, below which anaerobic energy flux is switched on and CR ratios become more exothermic than the oxycaloric equivalent. plim may be significanlty below the critical pO2. |
| Linearity | Linearity is the ability of the method to produce test results that are proportional, either directly or by a well-defined mathematical transformation, to the concentration of the analyte in samples within a given range. This property is inherent in the Beer-Lambert law for absorbance alone, but deviations occur in scattering media. It is also a property of fluorescence, but a fluorophore may not exhibit linearity, particularly over a large range of concentrations. | |
| Living cells | ce | Cell viability in living cells should be >95 % for various experimental investigations, including cell respirometry. Viable cells (vce) are characterized by an intact plasma membrane barrier function. The total cell count (Nce) is the sum of viable cells (Nvce) and dead cells (Ndce). In contrast, the plasma membrane can be permeabilized selectively by mild detergents (digitonin), to obtain the mt-preparation of permeabilized cells used for cell ergometry. Living cells are frequently labelled as intact cells in the sense of the total cell count, but intact may suggest dual meanings of viable or unaffected by a disease or mitochondrial injury. |
| Malate-aspartate shuttle | The malate-aspartate shuttle involves the glutamate-aspartate carrier and the 2-oxoglutarate carrier exchanging malate2- for 2-oxoglutarate2-. Cytosolic and mitochondrial malate dehydrogenase and transaminase complete the shuttle for the transport of cytosolic NADH into the mitochondrial matrix. It is most important in heart, liver and kidney. | |
| Microplates | Microplate readers allow large numbers of sample reactions to be assayed in well format microtitre plates. The most common microplate format used in academic research laboratories or clinical diagnostic laboratories is 96-well (8 by 12 matrix) with a typical reaction volume between 100 and 200 µL per well. a wide range of applications involve the use of fluorescence measurements , although they can also be used in conjunction with absorbance measurements. | |
| Mitochondrial marker | mt-marker | Mitochondrial markers are structural or functional properties that are specific for mitochondria. A structural mt-marker is the area of the inner mt-membrane or mt-volume determined stereologically, which has its limitations due to different states of swelling. If mt-area is determined by electron microscopy, the statistical challenge has to be met to convert area into a volume. When fluorescent dyes are used as mt-marker, distinction is necessary between mt-membrane potential dependent and independent dyes. mtDNA or cardiolipin content may be considered as a mt-marker. Mitochondrial marker enzymes may be determined as molecular (amount of protein) or functional properties (enzyme activities). Respiratory capacity in a defined respiratory state of a mt-preparation can be considered as a functional mt-marker, in which case respiration in other respiratory states is expressed as flux control ratios. » MiPNet article |
| Mitochondrial membrane potential | mtMP, ΔΨp+, ΔelFep+ [V] | The mitochondrial membrane potential difference, mtMP or ΔΨp+ = ΔelFep+, is the electric part of the protonmotive force, Δp = ΔmFeH+.
ΔΨp+ is the potential difference across the mitochondrial inner membrane (mtIM), expressed in the electric unit of volt [V]. Electric force of the mitochondrial membrane potential is the electric energy change per ‘motive’ charge or per charge moved across the transmembrane potential difference, with the number of ‘motive’ charges expressed in the unit coulomb [C]. |
| Mitochondrial respiration | Integrative measure of the dynamics of complex coupled metabolic pathways, including metabolite transport across the mt-membranes, TCA cycle function with electron transfer through dehydrogenases in the mt-matrix, membrane-bound electron transfer mET-pathway, the transmembrane proton circuit, and the phosphorylation system. | |
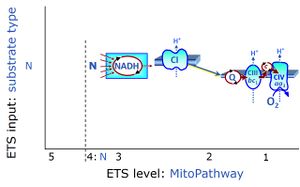
| N-junction |
The N-junction is a junction for convergent electron flow in the electron transfer pathway (ET-pathway) from type N substrates (further details »N-pathway control state) through the mt-NADH pool to Complex I (CI), and further transfer through the Q-junction to Complex III (CIII). Representative type N substrates are pyruvate (P), glutamate (G) and malate (M). The corresponding dehydrogenases (PDH, GDH, MDH) and some additional TCA cycle dehydrogenases (isocitrate dehydrogenase, oxoglutarate dehydrogenase generate NADH, the substrate of Complex I (CI). The concept of the N-junction and F-junction provides a basis for defining categories of SUIT protocols based on Electron-transfer-pathway states. | |
| NADH calibration - DatLab | NADH calibration | |
| NADH electron transfer-pathway state | N |
The NADH electron transfer-pathway state (N) is obtained by addition of NADH-linked substrates (CI-linked), feeding electrons into the N-junction catalyzed by various mt-dehydrogenases. N-supported flux is induced in mt-preparations by the addition of NADH-generating substrate combinations of pyruvate (P), glutamate (G), malate (M), oxaloacetate (Oa), oxoglutarate (Og), citrate, hydroxybutyrate. These N-junction substrates are (indirectly) linked to Complex I by the corresponding dehydrogenase-catalyzed reactions reducing NAD+ to NADH+H+ + H+. The most commonly applied N-junction substrate combinations are: PM, GM, PGM. The malate-anaplerotic pathway control state (M alone) is a special case related to malic enzyme (mtME). The glutamate-anaplerotic pathway control state (G alone) supports respiration through glutamate dehydrogenase (mtGDH). Oxidation of tetrahydrofolate is a NAD(P)H linked pathway with formation of formate. In mt-preparations, succinate dehydrogenase (SDH; CII) is largely substrate-limited in N-linked respiration, due to metabolite depletion into the incubation medium. The residual involvement of S-linked respiration in the N-pathway control state can be further suppressed by the CII-inhibitor malonic acid). In the N-pathway control state ET pathway level 4 is active. |
| NS-S pathway control efficiency | jNS-S | The NS-S pathway control efficiency expresses the relative stimulation of succinate supported respiration (S) by NADH-linked substrates (N), with the S-pathway control state as the background state and the NS-pathway control state as the reference state. In typical SUIT protocols with type N and S substrates, flux in the NS-pathway control state NS is inhibited by rotenone to measure flux in the S-pathway control state, S(Rot) or S. Then the NS-S pathway control efficiency in the ET-coupling state is j(NS-S)E = (NSE-SE)/NSE The NS-S pathway control efficiency expresses the fractional change of flux in a defined coupling-control state when inhibition by rotenone is removed from flux under S-pathway control in the presence of a type N substrate combination. Experimentally rotenone Rot is added to the NS-state. The reversed protocol, adding N-substrates to a S-pathway control background does not provide a valid estimation of S-respiration with succinate in the absence of Rot, since oxaloacetate accumulates as a potent inhibitor of succinate dehydrogenase CII. |
| Nigericin | Nigericin is a H+/K+ antiporter, which allows the electroneutral transport of these two ions in opposite directions across the mitochondrial inner membrane following the K+ concentration gradient. In the presence of K+, nigericin decreases pH in the mitchondrial matrix, thus, almost fully collapses the transmembrane ΔpH, which leads to the compensatory increase of the electric mt-membrane potential. Therefore, it is ideal to use to dissect the two components of the protonmotive force, ΔpH and mt-membrane potential. It is recommended to use the lowest possible concentration of nigericin, which creates a maximal mitochondrial hyperpolarization. In the study of Komlodi 2018 J Bioenerg Biomembr, 20 nM was applied on brain mitochondria isolated from guinea-pigs using 5 mM succinate in the LEAK state which caused maximum hyperpolarisation, but did not fully dissipate the transmembrane ΔpH. Other groups (Selivanov et al 2008; Lambert et al 2004), however, used 100 nM nigericin, which in their hands fully collapsed transmembrane ΔpH using succinate as a respiratory substrate on isolated rat brain and skeletal muscle in the LEAK state. | |
| Noise | In fluorometry and spectrophotometry, noise can be attributed to the statistical nature of the photon emission from a light source and the inherent noise in the instrument’s electronics. The former causes problems in measurements involving samples of analytes with a low extinction coefficient and present only in low concentrations. The latter becomes problematic with high absorbance samples where the light intensity emerging from the sample is very small. | |
| Noncoupled respiration | E |
Noncoupled respiration is maximum electron flow in an open-transmembrane proton circuit mode of operation (see ET capacity). » MiPNet article |
| Normalization of rate | Normalization of rate (respiratory rate, rate of hydrogen peroxide production, growth rate) is required to report experimental data. Normalization of rates leads to a diversity of formats. Normalization is guided by physicochemical principles, methodological considerations, and conceptual strategies. The challenges of measuring respiratory rate are matched by those of normalization. Normalization of rates for: (1) the number of objects (cells, organisms); (2) the volume or mass of the experimental sample; and (3) the concentration of mitochondrial markers in the instrumental chamber are sample-specific normalizations, which are distinguished from system-specific normalization for the volume of the instrumental chamber (the measuring system). Metabolic flow, I, per countable object increases as the size of the object is increased. This confounding factor is eliminated by expressing rate as sample-mass specific or sample-volume specific flux, J. Flow is an extensive quantity, whereas flux is a specific quantity. If the aim is to find differences in mitochondrial function independent of mitochondrial density, then normalization to a mitochondrial marker is imperative. Flux control ratios and flux control efficiencies are based on internal normalization for rate in a reference state, are independent of externally measured markers and, therefore, are statistically robust. | |
| Nuclear respiratory factor 1 | NRF-1 | Nuclear respiratory factor 1 is a transcription factor downstream of PGC-1alpha involved in coordinated expression of nDNA and mtDNA. |
| O2k | O2k | O2k - Oroboros O2k: the modular system for high-resolution respirometry. |
| O2k-FluoRespirometer | The Oroboros O2k-FluoRespirometer - the experimental system complete for high-resolution respirometry (HRR), including fluorometry, the TIP2k and the O2k-sV-Module allowing simultaneous monitoring of oxygen consumption together with either ROS production (AmR), mt-membrane potential (TMRM, Safranin and Rhodamine 123), Ca2+ (CaG) or ATP production (MgG). The O2k-FluoRespirometer supports all add-on O2k-Modules: O2k-TPP+ ISE-Module, O2k-pH ISE-Module, O2k-NO Amp-Module, enabling measurement of mt-membrane potential with ion sensitive electrodes (ISE for TPP+ or TPMP+) or pH. | |
| O2k-sV-Module | O2k-sV-Module |
The O2k-sV-Module is the O2k small-volume module, comprised of two Duran® glass chambers of 12 mm inner diameter specifically developed to perform high-resolution respirometry with reduced amounts of biological sample, and all the components necessary for a smaller operation volume V of 0.5 mL. The current DatLab version is included in the delivery of this revolutionary module. |
| OXPHOS capacity | P | |
| Octanoate | Oca | Octanoate (octanoic acid). C8H16O2 Common name: Caprylic acid. |
| Open system | An open system is a system with boundaries that allow external exchange of energy and matter; the surroundings are merely considered as a source or sink for quantities transferred across the system boundaries (external flows, Iext). | |
| Oroboros Instruments Corp |
| |
| Oxidative phosphorylation | OXPHOS | |
| Oxycaloric equivalent | DeltakHO2 | The oxycaloric equivalent is the theoretically derived enthalpy change of the oxidative catabolic reactions per amount of oxygen respired, DeltakHO2, ranging from -430 to -480 kJ/mol O2. The oxycaloric equivalent is used in indirect calorimetry to calculate the theoretically expected metabolic heat flux from the respirometrically measured metabolic oxygen flux. Calorimetric/respirometric ratios (CR ratios; heat/oxygen flux ratios) are experimentally determined by calorespirometry. A CR ratio more exothermic than the oxycaloric equivalent of -480 kJ/mol indicates the simultaneous involvement of aerobic and anaerobic mechanisms of energy metabolism. |
| Oxygen calibration - DatLab | O2 calibration is the calibration in DatLab of the oxygen sensor. It is a prerequisite for obtaining accurate measurements of respiration. Accurate calibration of the oxygen sensor depends on (1) equilibration of the incubation medium with air oxygen partial pressure at the temperature defined by the experimenter; (2) zero oxygen calibration; (3) high stability of the POS signal tested for sufficiently long periods of time; (4) linearity of signal output with oxygen pressure in the range between oxygen saturation and zero oxygen pressure; and (5) accurate oxygen solubility for aqueous solutions for the conversion of partial oxygen pressure into oxygen concentration. The standard oxygen calibration procedure is described below for high-resolution respirometry with the calibration routine using instrumental calibration DL-Protocols in DatLab. | |
| Oxygen flow | IO2 [mol·s-1] or [mol·s-1·x-1] | Respiratory oxygen flow is the oxygen consumption per total system, which is an extensive quantity. Flow is advancement of a transformation in a system per time [mol·s-1], when 'system' is defined as the experimental system (e.g. an open or closed chamber). Flow is distinguished from the size-specific quantity flux obtained by normalization of flow per volume of the experimental system [mol·s-1·m-3]. An experimental object, e.g. a living cell, may be considered as the 'experimental system'. Then oxygen flow per cell has the unit [mol·s-1·x-1], where [x] is the elementary unit for a count. Oxygen flow or respiration per cell [amol·s-1·x-1] = [pmol·s-1·Mx-1] is normalized for the cell count, distinguished from oxygen flux (e.g. per mg protein or wet mass). These are different forms of normalization of rate. |
| Oxygen flux | JO2 | Oxygen flux, JO2, is a specific quantity. Oxygen flux is oxygen flow, IO2 [mol·s-1 per system] (an extensive quantity), divided by system size. Flux may be volume-specific (flow per volume [pmol·s-1·mL-1]), mass-specific (flow per mass [pmol·s-1·mg-1]), or marker-specific (flow per mtEU). Oxygen flux (e.g., per body mass, or per cell volume) is distinguished from oxygen flow (per number of objects, such as cells), IO2 [mol·s-1·x-1]. These are different forms of normalization of rate. |
| Oxygen flux - instrumental background | J°O2 | Instrumental background oxygen flux, J°O2, in a respirometer is due to oxygen consumption by the POS, and oxygen diffusion into or out of the aqueous medium in the O2k-chamber. It is a property of the instrumental system, measured in the range of experimental oxygen levels by a standardized instrumental O2 background test. The oxygen regime from air saturation towards zero oxygen is applied generally in experiments with isolated mitochondria, and living or permeabilized cells. To overcome oxygen diffusion limitation in permeabilized fibers and homogenates, an elevated oxygen regime is applied, requiring instrumental background test in the same range of elevated oxygen. |
| Oxygen kinetics | Oxygen kinetics describes the dependence of respiration of isolated mitochondria or cells on oxygen partial pressure. Frequently, a strictly hyperbolic kinetics is observed, with two parameters, the oxygen pressure at half-maximum flux, p50, and maximum flux, Jmax. The p50 is in the range of 0.2 to 0.8 kPa for cytochrome c oxidase, isolated mitochondria and small cells, strongly dependent on Jmax and coupling state. | |
| Oxygen pressure | pO2 [kPa] | Oxygen pressure or partial pressure of oxygen [kPa], related to oxygen concentration in solution by the oxygen solubility, SO2 [µM/kPa]. |
| Oxygen signal | The oxygen signal of the Oroboros O2k is transmitted from the electrochemical polarographic oxygen sensor (OroboPOS) for each of the two O2k-chambers to DatLab. The primary signal is a current [µA] which is converted into a voltage [V] (raw signal), and calibrated in SI units for amount of substance concentration [µmol·L-1 or µM]. For technical reasons, the raw signal is given in [V] (DatLab 7 and previous) or [µA] (DatLab 8). The value of the raw signal is the same, independent of the displayed unit ([V] or [µA]). In the following sections, only [µA] is used for information on the raw signal, but the same values in [V] apply for the raw signal when using DL7 or previous versions. | |
| Oxygen solubility | SO2 [µM/kPa] | The oxygen solubility, SO2 [µM/kPa] = [(µmol·L-1)/kPa], expresses the oxygen concentration in solution in equilibrium with the oxygen pressure in a gas phase, as a function of temperature and composition of the solution. The inverse of oxygen solubility is related to the activity of dissolved oxygen. The oxygen solubility in solution, SO2(aq), depends on temperature and the concentrations of solutes in solution, whereas the dissolved oxygen concentration at equilibrium with air, cO2*(aq), depends on SO2(aq), barometric pressure and temperature. SO2(aq) in pure water is 10.56 µM/kPa at 37 °C and 12.56 µM/kPa at 25 °C. At standard barometric pressure (100 kPa), cO2*(aq) is 207.3 µM at 37 °C (19.6 kPa partial oxygen pressure) or 254.7 µM at 25 °C (20.3 kPa partial oxygen pressure). In MiR05 and serum, the corresponding saturation concentrations are lower due to the oxygen solubility factor: 191 and 184 µM at 37 °C or 234 and 227 µM at 25 °C. |
| Oxygen solubility factor | FM | The oxygen solubility factor of the incubation medium, FM, expresses the effect of the salt concentration on oxygen solubility relative to pure water. In mitochondrial respiration medium MiR05, MiR05-Kit and MiR06, FM is 0.92 (determined at 30 and 37 °C) and in culture media is 0.89 (at 37 °C). FM varies depending on the temperature and composition of the medium. To determine the FM based on the oxygen concentration, specific methods and equipment are needed (see references Rasmussen HN, Rasmussen UF 2003 in MiPNet06.03). For other media, FM may be estimated using Table 4 in MiPNet06.03. For this purpose KCl based media can be described as "seawater" of varying salinity. The original data on sucrose and KCl-media (Reynafarje et al 1985), however, have been critizesed as artefacts and the FM of 0.92 is suggested in the temperature range of 10 °C to 40 °C as for MiR05. |
| P-L control efficiency | jP-L | |
| P/E control ratio | P/E | |
| P50 | p50 | p50 is the oxygen partial pressure at which (a) respiratory flux is 50% of maximum oxygen flux, Jmax, at saturating oxygen levels. The oxygen affinity is indirectly proportional to the p50. The p50 depends on metabolic state and rate. (b) p50 is the oxygen partial pressure at which oxygen binding (on myoglobin, haemoglobin) is 50%, or desaturation is 50%. |
| PB-Module | The PB-Module has been developed for conducting measurements of PhotoBiology, including photosynthesis. It consists of the PB Light Source and electronic components which are an integral part of the NextGen-O2k. Measurements are recorded and evaluated with the DatLab 8 software. | |
| PH calibration buffers | pH calibration buffers are prepared to obtain two or more defined pH values for calibration of pH electrodes and pH indicator dyes. | |
| POS calibration - dynamic | Calibration of the sensor response time. See also POS calibration - static. | |
| POS calibration - static | F5 | Two-point calibration of the polarographic oxygen sensor, comprising Air calibration and Zero calibration. See also POS calibration - dynamic. |
| Pathway control ratio | FCR | Substrate control ratios are flux control ratios FCR, at a constant mitochondrial coupling-control state. Whereas there are only three well-defined coupling-control states of mitochondrial respiration, L, P, E (LEAK respiration, OXPHOS, Electron transfer pathway), numerous Electron-transfer-pathway states are possible. Careful selection of the reference state, Jref, is required, for which some guidelines may be provided without the possibility to formulate general rules. FCR are best defined by taking Jref as the maximum flux (e.g. NSE), such that flux in various other respiratory states, Ji, is smaller or equal to Jref. However, this is not generally possible with FCR. For instance, the N/S pathway control ratio (at constant coupling-control state) may be larger or smaller than 1.0, depending on the mitochondrial source and various mitochondrial injuries. The S-pathway control state may be selected preferentially as Jref, if mitochondria with variable N-linked injuries are studied. In contrast, the reference state, Z, is strictly defined for flux control efficiency. |
| Photodecomposition | PD | Photodecomposition or photodegradation is the process of decay of organic material induced by increasing light intensity. Under aerobic conditions, the enhancement of photodecomposition by light intensity can be quantified by oxygen consumption in a controlled light regime. |
| Physiological pathway-control state | See Electron-transfer-pathway state. | |
| Polyether ether ketone | PEEK | Polyether ether ketone (PEEK) is a semicrystalline organic polymer thermoplastic, which is chemically very resistant, with excellent mechanical properties. PEEK is compatible with ultra-high vacuum applications, and its resistance against oxygen diffusion make it an ideal material for high-resolution respirometry (POS insulation; coating of stirrer bars; stoppers for closing the O2k-Chamber). |
| Polyvinylidene fluoride | PVDF | Polyvinylidene fluoride (PVDF) is a pure thermoplastic fluoropolymer, which is chemically very resistant, with excellent mechanical properties. It is used generally in applications requiring the highest purity, strength, and resistance to solvents, acids, bases and heat (Wikipedia). PVDF is resistant against oxygen diffusion which makes it an ideal material for high-resolution respirometry (coating of stirrer bars; stoppers for closing the O2k-Chamber). |
| Power O2k-FluoRespirometer | Power O2k-FluoRespirometer - optional configuration as additional system for increasing output combined with the O2k-FluoRespirometer (O2k-Series H). The Power O2k-FluoRespirometer includes the TIP2k and the O2k-sV-Module, and supports all add-on O2k-Modules of the Oroboros O2k. It can be added to an existing Oroboros O2k of any O2k-Series. This application does not require an additional ISS-Integrated Suction System and O2k-Titration Set. Furthermore, the OroboPOS-Mounting Tool of the OroboPOS Service Tools can be used from the available O2k and is not included. | |
| Power O2k-Respirometer | The Power O2k-Respirometer is an economical option for using additional O2k-Units to increase output in high-resolution respirometry. |