Difference between revisions of "SUIT-013"
From Bioblast
(Created page with "{{MitoPedia |abbr=NS(GM) |description= |info='''A''' 100px|link=http://wiki.oroboros.at/images/6/64/SUIT-013.pdf|Bioblast pdf »[http://wiki.oroboros.at/index...") |
|||
| (16 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr= | |abbr=ce | ||
|description= | |description=[[File:SUIT013 AmR ce D023.png|300px]] | ||
|info='''A''' | |info='''A''' | ||
}} | }} | ||
::: '''[[Categories of SUIT protocols |SUIT-category]]:''' ce | |||
::: '''[[Categories of SUIT protocols |SUIT-category]]:''' | ::: '''[[SUIT protocol pattern]]:''' ce1 | ||
::: '''[[SUIT protocol pattern]]:''' | SUIT-013 has been designed to study the oxygen dependence of H<sub>2</sub>O<sub>2</sub> production in intact cells. SUIT-013 AmR ce D023 specifically has been tested for demonstration of the oxygen dependence in baker´s yeast. For more details see: [[Komlodi 2021 MitoFit AmR-O2]]. The respiration medium has to be choosen carefully since the [[Amplex UltraRed]] assay leads to artefact formation with the components of the buffer. | ||
__TOC__ | __TOC__ | ||
Communicated by [[Komlodi T]] (last update | Communicated by [[Komlodi T]] and [[Gnaiger E]] (last update 2021-11-09) | ||
== DLP | == Specific DLP protocols == | ||
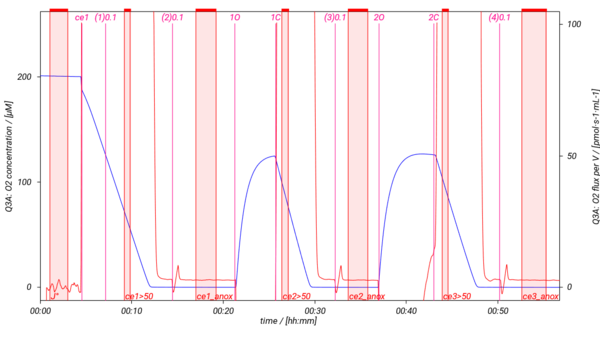
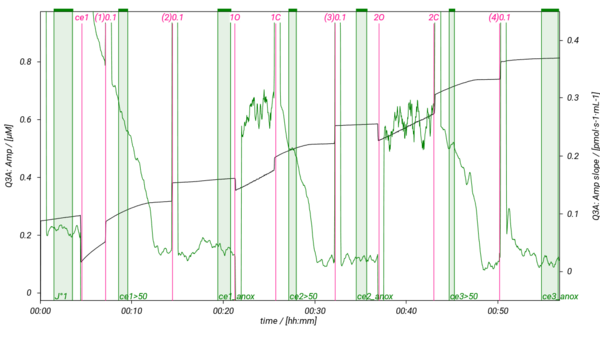
=== SUIT-013 AmR ce D023 === | |||
== | [[File:SUIT013 AmR ce D023.png|200px]] [[File:SUIT-013 O2 D023.png|600px]] | ||
[[File:SUIT-013 AmR D023.png|600px]] | |||
:::*[[SUIT-013 AmR ce D023]] for intact cells | |||
{{Template:SUIT-013}} | {{Template:SUIT-013}} | ||
== Strengths and limitations == | == Strengths and limitations == | ||
:::* To study the inhibitory effect of the AmR assay on the respiration using living cells, the following protocols are recommended: [[SUIT 003 AmR ce D058]] with AmR and [[SUIT 003 AmR ce D059]] with carrier titration as a control. These two protocols have to be done in parallel. | |||
:::* | |||
:::+ Reasonable duration of the experiment. | :::+ Reasonable duration of the experiment. | ||
:::+ | :::+ Quick demonstration of the oxygen dependence of H<sub>2</sub>O<sub>2</sub> production in intact cells. | ||
:::+ No externally added substrates are required with yeast. | |||
::: | |||
== Compare SUIT protocols == | == Compare SUIT protocols == | ||
== References == | |||
{{#ask:[[Category:Publications]] [[Instrument and method::O2k-Protocol]] [[Additional label::SUIT-013]] | |||
|?Was published in year=Year | |||
|?Has title=Reference | |||
|?Mammal and model=Organism | |||
|?Tissue and cell=Tissue;cell | |||
|format=broadtable | |||
|limit=5000 | |||
|offset=0 | |||
|sort=Was published in year | |||
|order=descending | |||
}} | |||
{{MitoPedia concepts | {{MitoPedia concepts | ||
| Line 46: | Line 43: | ||
}} | }} | ||
{{MitoPedia methods | {{MitoPedia methods | ||
|mitopedia method= | |mitopedia method=Fluorometry | ||
}} | }} | ||
Latest revision as of 09:38, 9 November 2021
Description
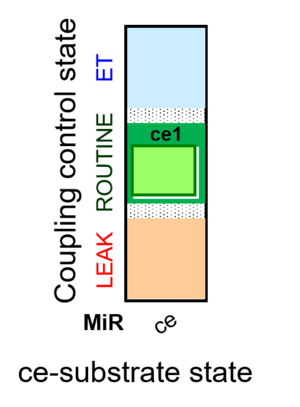
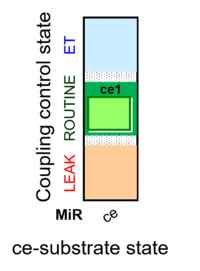
Abbreviation: ce
Reference: A
- SUIT-category: ce
- SUIT protocol pattern: ce1
SUIT-013 has been designed to study the oxygen dependence of H2O2 production in intact cells. SUIT-013 AmR ce D023 specifically has been tested for demonstration of the oxygen dependence in baker´s yeast. For more details see: Komlodi 2021 MitoFit AmR-O2. The respiration medium has to be choosen carefully since the Amplex UltraRed assay leads to artefact formation with the components of the buffer.
Communicated by Komlodi T and Gnaiger E (last update 2021-11-09)
Specific DLP protocols
SUIT-013 AmR ce D023
- SUIT-013 AmR ce D023 for intact cells
Steps and respiratory states
| Step | State | Pathway | Q-junction | Comment - Events (E) and Marks (M) |
|---|---|---|---|---|
| 0DTPA |
| |||
| 0SOD |
| |||
| 0HRP |
| |||
| 0AmR |
|
| Step | State | Pathway | Q-junction | Comment - Events (E) and Marks (M) |
|---|---|---|---|---|
| ce1 | ROUTINE | ce1
|
- Bioblast links: SUIT protocols - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Coupling control
- Pathway control
- Main fuel substrates
- » Glutamate, G
- » Glycerophosphate, Gp
- » Malate, M
- » Octanoylcarnitine, Oct
- » Pyruvate, P
- » Succinate, S
- Main fuel substrates
- Glossary
Strengths and limitations
- To study the inhibitory effect of the AmR assay on the respiration using living cells, the following protocols are recommended: SUIT 003 AmR ce D058 with AmR and SUIT 003 AmR ce D059 with carrier titration as a control. These two protocols have to be done in parallel.
- + Reasonable duration of the experiment.
- + Quick demonstration of the oxygen dependence of H2O2 production in intact cells.
- + No externally added substrates are required with yeast.
Compare SUIT protocols
References
| Year | Reference | Organism | Tissue;cell | |
|---|---|---|---|---|
| MiPNet24.10 H2O2 flux analysis | 2021-10-22 | Hydrogen peroxide flux analysis using Amplex UltraRed assay in MiR05-Kit with DatLab 7.4 | ||
| Komlodi 2021 BEC AmR-O2 | 2021 | Komlódi T, Sobotka O, Gnaiger E (2021) Facts and artefacts on the oxygen dependence of hydrogen peroxide production using Amplex UltraRed. Bioenerg Commun 2021.4. https://doi.org/10.26124/bec:2021-0004 | Saccharomyces cerevisiae | Other cell lines |
| MiPNet20.14 AmplexRed H2O2-production | 2019-06-24 | O2k-FluoRespirometry: HRR and simultaneous determination of H2O2 production with Amplex UltraRed. | Mouse | Heart |
| Komlodi 2018 Methods Mol Biol | 2018 | Komlodi T, Sobotka O, Krumschnabel G, Bezuidenhout N, Hiller E, Doerrier C, Gnaiger E (2018) Comparison of mitochondrial incubation media for measurement of respiration and hydrogen peroxide production. Methods Mol Biol 1782:137-55. | Human Mouse | Skeletal muscle HEK |
| Makrecka-Kuka 2015 Biomolecules | 2015 | Makrecka-Kuka M, Krumschnabel G, Gnaiger E (2015) High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. https://doi.org/10.3390/biom5031319 | Human Mouse | Heart Nervous system HEK |
| Krumschnabel 2015 Methods Mol Biol | 2015 | Krumschnabel G, Fontana-Ayoub M, Sumbalova Z, Heidler J, Gauper K, Fasching M, Gnaiger E (2015) Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Methods Mol Biol 1264:245-61. | Mouse | Nervous system |
MitoPedia concepts:
MiP concept,
SUIT protocol,
Recommended
MitoPedia methods:
Fluorometry