Difference between revisions of "Nicholls 2013 Academic Press"
From Bioblast
| Line 13: | Line 13: | ||
:::: ''"Thermodynamic ignorance is also responsible for some extraordinary errors found in the current literature, particularly in the field of mitochondrial physiology (see Part 3)."'' - Nicholls, Ferguson (2013): Part 3 (p 27). | :::: ''"Thermodynamic ignorance is also responsible for some extraordinary errors found in the current literature, particularly in the field of mitochondrial physiology (see Part 3)."'' - Nicholls, Ferguson (2013): Part 3 (p 27). | ||
::::* Part 3 suffers from a confusion between ''system'' and ''process'' (reaction, diffusion, transformation in general) throughout the text. | ::::* <span style="color:#FF0000"> Part 3 suffers from a confusion between ''system'' and ''process'' (reaction, diffusion, transformation in general) throughout the text. | ||
::::* p. 27: Distinction of three types of thermodynamic systems (isolated, closed, open) is insufficient in the context of vectorial metabolism and the protonmotive force. For explaining the nature of the protonmotive force, we need to introduce further categories of systems: [[homogenous system]]s, [[continuous system]]s with gradients, and [[compartmental system]]s with discontinuities across compartmental boundaries (discontinuous or heterogenous systems). | ::::* p. 27: <span style="color:#FF0000"> Distinction of three types of thermodynamic systems (isolated, closed, open) is insufficient in the context of vectorial metabolism and the protonmotive force. For explaining the nature of the protonmotive force, we need to introduce further categories of systems: [[homogenous system]]s, [[continuous system]]s with gradients, and [[compartmental system]]s with discontinuities across compartmental boundaries (discontinuous or heterogenous systems). | ||
::::* p. 27: Open systems: The | ::::* p. 27: Open systems: <span style="color:#FF0000"> The opinion that </span style> ''"classical equilibrium thermodynamics cannot be applid precisely to open systems because the flow or matter across their boundaries precludes the establishment of a true equilibrium"'' <span style="color:#FF0000"> is in direct contradiction to the presentation of Figure 3.2, which attempts to explain the Gibbs energy of reaction by states maintained </span style> ''"by continuously supplying substrate and removing product"'' (p. 31). | ||
::::* p. 28: ''"It is this displacement from equilibrium that defines the capacity of the reaction to perform useful work."'' - The term ''capacity'' is consfused with the term ''potential''. | ::::* p. 28: ''"It is this displacement from equilibrium that defines the capacity of the reaction to perform useful work."'' - <span style="color:#FF0000"> The term ''capacity'' is consfused with the term ''potential''. | ||
::::* p. 29: ''".. the driving force for a reaction is an increase in entropy .." - Entropy [J·K<sup>-1</sup>] = driving force | ::::* p. 29: ''".. the driving force for a reaction is an increase in entropy .." - <span style="color:#FF0000"> Entropy [J·K<sup>-1</sup>] = driving force? | ||
::::* p. 29: ''"The | ::::* p. 29: ''"The thermodynamic function that takes account of this enthalpy flow is the Gibbs energy change, Δ''G'', which is the quantitative measure of the net driving force (at constant temperature and pressure).''" - <span style="color:#FF0000"> Gibbs energy = net driving force ? There is a lot of confusion to be removed. What is ''enthalpy flow''? | ||
::::* p. | ::::* p. 29: ''"The available energy in a gradient of ions is quantified by a further variant of the Gibbs energy change, namely the ion electrochemical gradient"'' - <span style="color:#FF0000"> Electrochemical gradient = Gibbs energy change? | ||
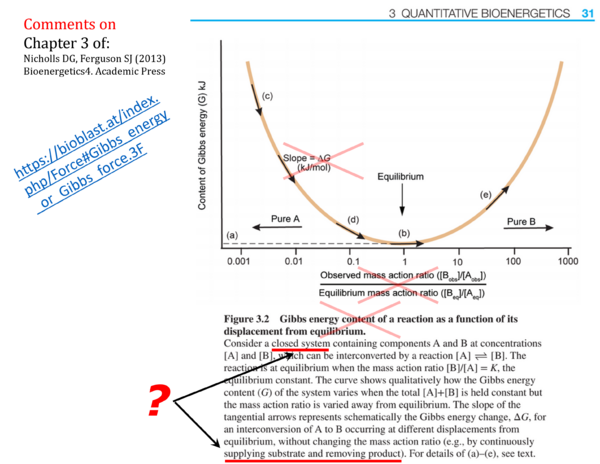
::::* p. 31 (Figure 3.2.): | [[File:Nicholls DG 2013 Fig 3.2 Gibbs energy.png|right|600px]] | ||
::::* p. 31 (Figure caption to Figure 3.2.): '''Gibbs energy content of a reaction as a function of its displacement from equilibrium''' - <span style="color:#FF0000"> The term ''Gibbs energy content of a reaction'' is not consistent with IUPAC nomenclature, and the meaning of a ''Gibbs energy content of a reaction'' is unclear. The Gibbs energy of a ''closed system'' (at constant temperature and pressure; the term ''content'' is superfluous in this context) is an extensive quantity [J] that changes as a function of [[advancement]] of a reaction (which is also an extensive quantity [mol]), but this does not relate to a Gibbs energy ''content of a reaction''. | |||
::::* p. 32: ''"Note again that Δ''G'' is a differential .."'' - As seen in Fig. 3.2, the symbol and meaning of a '''differential''' are not understood. | ::::* p. 31 (Figure 3.2.): <span style="color:#FF0000"> The figure presents an inconsistency of units. The </span style> 'content of Gibbs energy (G) kJ' <span style="color:#FF0000"> is plotted on the ''Y''-axis, and the ''X''-axis is a dimensionless ''ratio''. The slope d''G''/d''ratio'', therefore, has to have the same unit as ''G'' [kJ]. Paradoxically, the Figure shows </span style> '''Slope = Δ''G'' (kJ/mol)'''. <span style="color:#FF0000"> Δ''G'' is not the "slope" in this figure. In a meaningful presentation of the concept of molar Gibbs energy change of reaction (Gibbs [[force]] [kJ/mol]) in a closed system at constant temperature and pressure, the Gibbs energy is plotted as a function of [[advancement]] of the reaction [mol]. | ||
::::* p. 32: ''"Note again that Δ''G'' is a differential .."'' - <span style="color:#FF0000"> As seen in Fig. 3.2, the symbol and meaning of a '''differential''' are not understood. | |||
== Cited by == | == Cited by == | ||
Revision as of 07:12, 5 July 2022
| Nicholls DG, Ferguson SJ (2013) Bioenergetics4. Academic Press 419 pp. |
Nicholls DG, Ferguson SJ (2013) Academic Press
Abstract:
Thermodynamic ignorance
- With reference to thermodynamic ignorance in Part 3, see Gibbs energy or Gibbs force?
Nicholls DG, Ferguson SJ (2013) Bioenergetics4. Academic Press
- "Thermodynamic ignorance is also responsible for some extraordinary errors found in the current literature, particularly in the field of mitochondrial physiology (see Part 3)." - Nicholls, Ferguson (2013): Part 3 (p 27).
- Part 3 suffers from a confusion between system and process (reaction, diffusion, transformation in general) throughout the text.
- p. 27: Distinction of three types of thermodynamic systems (isolated, closed, open) is insufficient in the context of vectorial metabolism and the protonmotive force. For explaining the nature of the protonmotive force, we need to introduce further categories of systems: homogenous systems, continuous systems with gradients, and compartmental systems with discontinuities across compartmental boundaries (discontinuous or heterogenous systems).
- p. 27: Open systems: The opinion that "classical equilibrium thermodynamics cannot be applid precisely to open systems because the flow or matter across their boundaries precludes the establishment of a true equilibrium" is in direct contradiction to the presentation of Figure 3.2, which attempts to explain the Gibbs energy of reaction by states maintained "by continuously supplying substrate and removing product" (p. 31).
- p. 28: "It is this displacement from equilibrium that defines the capacity of the reaction to perform useful work." - The term capacity is consfused with the term potential.
- p. 29: ".. the driving force for a reaction is an increase in entropy .." - Entropy [J·K-1] = driving force?
- p. 29: "The thermodynamic function that takes account of this enthalpy flow is the Gibbs energy change, ΔG, which is the quantitative measure of the net driving force (at constant temperature and pressure)." - Gibbs energy = net driving force ? There is a lot of confusion to be removed. What is enthalpy flow?
- p. 29: "The available energy in a gradient of ions is quantified by a further variant of the Gibbs energy change, namely the ion electrochemical gradient" - Electrochemical gradient = Gibbs energy change?
- p. 31 (Figure caption to Figure 3.2.): Gibbs energy content of a reaction as a function of its displacement from equilibrium - The term Gibbs energy content of a reaction is not consistent with IUPAC nomenclature, and the meaning of a Gibbs energy content of a reaction is unclear. The Gibbs energy of a closed system (at constant temperature and pressure; the term content is superfluous in this context) is an extensive quantity [J] that changes as a function of advancement of a reaction (which is also an extensive quantity [mol]), but this does not relate to a Gibbs energy content of a reaction.
- p. 31 (Figure 3.2.): The figure presents an inconsistency of units. The 'content of Gibbs energy (G) kJ' is plotted on the Y-axis, and the X-axis is a dimensionless ratio. The slope dG/dratio, therefore, has to have the same unit as G [kJ]. Paradoxically, the Figure shows Slope = ΔG (kJ/mol). ΔG is not the "slope" in this figure. In a meaningful presentation of the concept of molar Gibbs energy change of reaction (Gibbs force [kJ/mol]) in a closed system at constant temperature and pressure, the Gibbs energy is plotted as a function of advancement of the reaction [mol].
- p. 32: "Note again that ΔG is a differential .." - As seen in Fig. 3.2, the symbol and meaning of a differential are not understood.
Cited by
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
Labels:
BEC 2020.2