Description
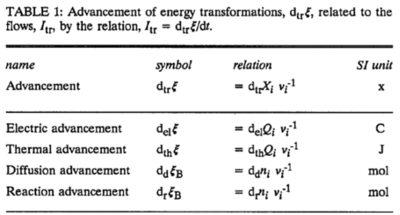
In an isomorphic analysis, any form of flow is the advancement of a process per unit of time, expressed in a specific motive unit [MU∙s-1], e.g., ampere for electric flow or current, Iel = delξ/dt [A≡C∙s-1], watt for thermal or heat flow, Ith = dthξ/dt [W≡J∙s-1], and for chemical flow of reaction, Ir = drξ/dt, the unit is [mol∙s-1] (extent of reaction per time). The corresponding motive forces are the partial exergy (Gibbs energy) changes per advancement [J∙MU-1], expressed in volt for electric force, ΔelF = ∂G/∂elξ [V≡J∙C-1], dimensionless for thermal force, ΔthF = ∂G/∂thξ [J∙J-1], and for chemical force, ΔrF = ∂G/∂rξ, the unit is [J∙mol-1], which deserves a specific acronym [Jol] comparable to volt [V]. For chemical processes of reaction (spontaneous from high-potential substrates to low-potential products) and compartmental diffusion (spontaneous from a high-potential compartment to a low-potential compartment), the advancement is the amount of motive substance that has undergone a compartmental transformation [mol]. The concept was originally introduced by De Donder [1]. Central to the concept of advancement is the stoichiometric number, νi, associated with each motive component i (transformant [2]).
In a chemical reaction r the motive entity is the stoichiometric amount of reactant, drni, with stoichiometric number νi. The advancement of the chemical reaction, drξ [mol], is defined as,
drξ = drni·νi-1
The flow of the chemical reaction, Ir [mol·s-1], is advancement per time,
Ir = drξ·dt-1
This concept of advancement is extended to compartmental diffusion and the advancement of charged particles [3], and to any discontinuous transformation in compartmental systems [2],
Abbreviation: dtrξ [MU]
Reference: Gnaiger (1993) Pure Appl Chem, Gnaiger 2020 BEC MitoPathways
Communicated by Gnaiger E (2018-11-02) last update 2021-01-27
Advancement versus amount
- In the chemical or molar format, advancement is expressed in the same unit as amount [mol]. Molar advancement and amount are distinguished as the dynamic expression related to rate and the static expression related to state. This is isomorphic to mechanical or geometric distance traveled (dynamic) compared to a 'distance measured' (static). You can run the distance of 400 m or 1000 m in a stadion of a constant distance measured per round. The advancement of a reaction may proceed independent of the amount of substrate or product contained in an open system. Substrates and products are tightly connected by the stoichiometric numbers in the advancement of a defined chemical reaction, but amounts of substrates and products have to be measured independently.
- "Unfortunately, using different symbols for extensive thermodynamic properties and their molar counterparts greatly increases the number of symbols and equations. .. Therefore, we will use a single set of symbols for thermodynamic quantities and let them represent the intensive, or molar, quantities" (p. 4; Alberty RA, Daniels F (1980) Physical chemistry. SI version. 5th ed, John Wiley & Sons, New York:692 pp.). - The unfortunate single set of symbols causes confusion on two different levels: (1) Molar quantities are not explicitly distinguished from extensive quantities. (2) Within the molar counterparts of the extensive quantities, there is no explicit distinction made between specific quantities (expressed per molar amount of substance; molar Gibbs energy) and intensive quantities (expressed per molar advancement; Gibbs force).
Advancement per volume
- The advancement of a transformation in a closed homogenous system (chemical reaction) or discontinuous system (diffusion) causes a change of concentration of substances i.
- The advancement causes a change of concentration due to a transformation, Δtrc, in contrast to a difference of concentrations calculated between difference states, Δc.
- » Advancement per volume, dtrY = dtrξ∙V-1
Synonyms
- advancement
- extent (of reaction)
- displacement (of a moving particle)
References
- De Donder T, Van Rysselberghe P (1936) Thermodynamic theory of affinity: a book of principles. Oxford, England: Oxford University Press:144 pp.
- Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65:1983-2002. - »Bioblast link«
- Prigogine I (1967) Introduction to thermodynamics of irreversible processes. Interscience New York, 3rd ed:147 pp. - »Bioblast link«
- Grosholz Emily R (2007) Representation and productive ambiguity in mathematics and the sciences. Oxford Univ Press 312 pp. - »Bioblast link« — ".. distance that is re-conceptualized to mean displacement, since we are instructed to suppose that a moving particle is traversing it."
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. https://doi.org/10.26124/bec:2020-0002
- Bioblast links: Force and membrane potential - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Fundamental relationships
- mt-Membrane potential and protonmotive force
- O2k-Potentiometry
- » O2k-Catalogue: O2k-TPP+ ISE-Module
- » O2k-Manual: MiPNet15.03 O2k-MultiSensor-ISE
- » TPP - O2k-Procedures: Tetraphenylphosphonium
- » Specifications: MiPNet15.08 TPP electrode
- » Poster
- » Unspecific binding of TPP+
- » TPP+ inhibitory effect
- O2k-Potentiometry
- O2k-Fluorometry
- » O2k-Catalogue: O2k-FluoRespirometer
- » O2k-Manual: MiPNet22.11 O2k-FluoRespirometer manual
- » Safranin - O2k-Procedures: MiPNet20.13 Safranin mt-membranepotential / Safranin
- » TMRM - O2k-Procedures: TMRM
- O2k-Fluorometry
- O2k-Publications
- Bioblast links: Normalization - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Quantities for normalization
- » Count in contrast to Number
- » Mitochondrial marker
- » O2k-Protocols: mitochondrial and marker-enzymes
- » Citrate synthase activity
- Quantities for normalization
- General
- Related keyword lists
MitoPedia concepts:
MiP concept,
Ergodynamics