Difference between revisions of "Flow"
| (2 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
|abbr=''I'' [MU∙s<sup>-1</sup>] | |abbr=''I'' [MU∙s<sup>-1</sup>] | ||
|description=In an isomorphic analysis, any form of '''flow''', ''I'' is the [[advancement]] of a process per unit of time, expressed in a specific motive unit [MU∙s<sup>-1</sup>], ''e.g.'', ampere for electric flow or current [A≡C∙s<sup>-1</sup>], watt for heat flow [W≡J∙s<sup>-1</sup>], and for chemical flow the unit is [mol∙s<sup>-1</sup>]. Flow is an [[extensive quantity]]. The corresponding isomorphic [[force]]s are the partial exergy (Gibbs energy) changes per advancement [J∙MU<sup>-1</sup>], expressed in volt for electric force [V≡J∙C<sup>-1</sup>], dimensionless for thermal force, and for chemical force the unit is [J∙mol<sup>-1</sup>], which deserves a specific acronym ([Jol]) comparable to volt. | |description=In an isomorphic analysis, any form of '''flow''', ''I'' is the [[advancement]] of a process per unit of time, expressed in a specific motive unit [MU∙s<sup>-1</sup>], ''e.g.'', ampere for electric flow or current [A≡C∙s<sup>-1</sup>], watt for heat flow [W≡J∙s<sup>-1</sup>], and for chemical flow the unit is [mol∙s<sup>-1</sup>]. Flow is an [[extensive quantity]]. The corresponding isomorphic [[force]]s are the partial exergy (Gibbs energy) changes per advancement [J∙MU<sup>-1</sup>], expressed in volt for electric force [V≡J∙C<sup>-1</sup>], dimensionless for thermal force, and for chemical force the unit is [J∙mol<sup>-1</sup>], which deserves a specific acronym ([Jol]) comparable to volt. | ||
|info=[[ | |info=[[BEC 2020.1]], [[Gnaiger_1993_Pure Appl Chem]] | ||
}} | }} | ||
<gallery heights="350px" mode="default" perrow="4" widths="350px"> | <gallery heights="350px" mode="default" perrow="4" widths="350px"> | ||
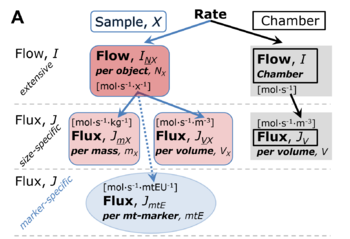
File:Rate.png |'''Normalization of rate.''' '''(A)''' Cell respiration is normalized for (1) the experimental '''Sample''' (flow per | File:Rate.png |'''Normalization of rate.''' '''(A)''' Cell respiration is normalized for (1) the experimental '''Sample''' (flow per [[count]], mass-specific flux, or cell-volume-specific flux); or (2) for the '''Chamber''' volume. Normalization yields the [[specific quantity]] ''[[flux]]'' from the [[extensive quantity]] ''flow''. From [[Gnaiger 2019 MitoFit Preprint Arch]]. | ||
</gallery> | </gallery> | ||
| Line 19: | Line 19: | ||
|order=ascending | |order=ascending | ||
}} | }} | ||
{{Template:Keywords: Normalization}} | |||
{{MitoPedia concepts | {{MitoPedia concepts | ||
|mitopedia concept=MiP concept, Ergodynamics | |mitopedia concept=MiP concept, Ergodynamics | ||
}} | }} | ||
Latest revision as of 12:49, 23 May 2020
Description
In an isomorphic analysis, any form of flow, I is the advancement of a process per unit of time, expressed in a specific motive unit [MU∙s-1], e.g., ampere for electric flow or current [A≡C∙s-1], watt for heat flow [W≡J∙s-1], and for chemical flow the unit is [mol∙s-1]. Flow is an extensive quantity. The corresponding isomorphic forces are the partial exergy (Gibbs energy) changes per advancement [J∙MU-1], expressed in volt for electric force [V≡J∙C-1], dimensionless for thermal force, and for chemical force the unit is [J∙mol-1], which deserves a specific acronym ([Jol]) comparable to volt.
Abbreviation: I [MU∙s-1]
Reference: BEC 2020.1, Gnaiger_1993_Pure Appl Chem
Normalization of rate. (A) Cell respiration is normalized for (1) the experimental Sample (flow per count, mass-specific flux, or cell-volume-specific flux); or (2) for the Chamber volume. Normalization yields the specific quantity flux from the extensive quantity flow. From Gnaiger 2019 MitoFit Preprint Arch.
References
| Bioblast link | Reference | Year |
|---|---|---|
| Gnaiger 1993 Hypoxia | Gnaiger E (1993) Efficiency and power strategies under hypoxia. Is low efficiency at high glycolytic ATP production a paradox? In: Surviving hypoxia: Mechanisms of control and adaptation. Hochachka PW, Lutz PL, Sick T, Rosenthal M, Van den Thillart G (eds) CRC Press, Boca Raton, Ann Arbor, London, Tokyo:77-109. | 1993 |
| Gnaiger 1993 Pure Appl Chem | Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65:1983-2002. http://dx.doi.org/10.1351/pac199365091983 | 1993 |
| Gnaiger 2020 BEC MitoPathways | Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | 2020 |
| BEC 2020.1 doi10.26124bec2020-0001.v1 | Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 | 2020 |
- Bioblast links: Normalization - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Quantities for normalization
- » Count in contrast to Number
- » Mitochondrial marker
- » O2k-Protocols: mitochondrial and marker-enzymes
- » Citrate synthase activity
- Quantities for normalization
- General
- Related keyword lists
MitoPedia concepts:
MiP concept,
Ergodynamics