Anoar 2021 Front Neurosci
| Anoar S, Woodling NS, Niccoli T (2021) Mitochondria dysfunction in frontotemporal dementia/amyotrophic lateral sclerosis: lessons from Drosophila models. Front Neurosci 15:786076. https://doi.org/10.3389/fnins.2021.786076 |
Anoar S, Woodling NS, Niccoli T (2021) Front Neurosci
Abstract: Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are neurodegenerative disorders characterized by declining motor and cognitive functions. Even though these diseases present with distinct sets of symptoms, FTD and ALS are two extremes of the same disease spectrum, as they show considerable overlap in genetic, clinical and neuropathological features. Among these overlapping features, mitochondrial dysfunction is associated with both FTD and ALS. Recent studies have shown that cells derived from patients' induced pluripotent stem cells (iPSC)s display mitochondrial abnormalities, and similar abnormalities have been observed in a number of animal disease models. Drosophila models have been widely used to study FTD and ALS because of their rapid generation time and extensive set of genetic tools. A wide array of fly models have been developed to elucidate the molecular mechanisms of toxicity for mutations associated with FTD/ALS. Fly models have been often instrumental in understanding the role of disease associated mutations in mitochondria biology. In this review, we discuss how mutations associated with FTD/ALS disrupt mitochondrial function, and we review how the use of Drosophila models has been pivotal to our current knowledge in this field.
• Bioblast editor: Gnaiger E
Labels:
Pathology: Neurodegenerative
Organism: Drosophila
Enzyme: Complex II;succinate dehydrogenase
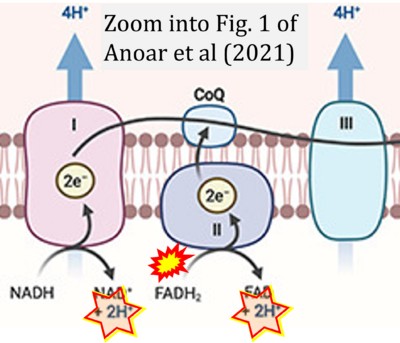
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«