Wang 2016 ACS Appl Mater Interfaces
| Wang G, Feng H, Gao A, Hao Q, Jin W, Peng X, Li W, Wu G, Chu PK (2016) Extracellular electron transfer from aerobic bacteria to Au-loaded TiO2 semiconductor without light: a new bacteria-killing mechanism other than localized surface plasmon resonance or microbial fuel cells. ACS Appl Mater Interfaces 8:24509-16. doi: 10.1021/acsami.6b10052 |
Wang G, Feng H, Gao A, Hao Q, Jin W, Peng X, Li W, Wu G, Chu PK (2016) ACS Appl Mater Interfaces
Abstract: Titania loaded with noble metal nanoparticles exhibits enhanced photocatalytic killing of bacteria under light illumination due to the localized surface plasmon resonance (LSPR) property. It has been shown recently that loading with Au or Ag can also endow TiO2 with the antibacterial ability in the absence of light. In this work, the antibacterial mechanism of Au-loaded TiO2 nanotubes (Au@TiO2-NT) in the dark environment is studied, and a novel type of extracellular electron transfer (EET) between the bacteria and the surface of the materials is observed to cause bacteria death. Although the EET-induced bacteria current is similar to the LSPR-related photocurrent, the former takes place without light, and no reactive oxygen species (ROS) are produced during the process. The EET is also different from that commonly attributed to microbial fuel cells (MFC) because it is dominated mainly by the materials' surface, but not the bacteria, and the environment is aerobic. EET on the Au@TiO2-NT surface kills Staphylococcus aureus, but if it is combined with special MFC bacteria, the efficiency of MFC may be improved significantly.
• Bioblast editor: Gnaiger E
Labels:
Enzyme: Complex II;succinate dehydrogenase
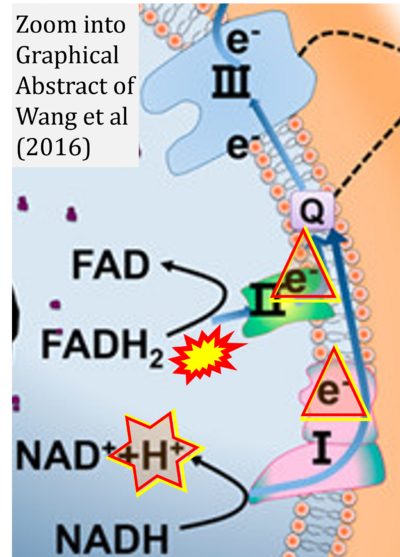
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«