Tseng 2022 Cells: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Publication | {{Publication | ||
|title=Tseng W-W, Wei A-C (2022) Kinetic | |title=Tseng W-W, Wei A-C (2022) Kinetic mathematical modeling of oxidative phosphorylation in cardiomyocyte mitochondria. Cells 11:4020. https://doi.org/10.3390/cells11244020 | ||
|info=[https://doi.org/10.3390/cells11244020 Open Access] | |info=[https://doi.org/10.3390/cells11244020 Open Access] | ||
|authors=Tseng W-W, Wei A-C | |authors=Tseng W-W, Wei A-C | ||
| Line 10: | Line 10: | ||
[[File:Tseng 2022 Cells CORRECTION.png|right|400px]] | [[File:Tseng 2022 Cells CORRECTION.png|right|400px]] | ||
{{Template:Correction FADH2 and S-pathway}} | {{Template:Correction FADH2 and S-pathway}} | ||
{{Template:Correction NAD and H+}} | |||
{{Labeling | {{Labeling | ||
|enzymes=Complex II;succinate dehydrogenase | |enzymes=Complex II;succinate dehydrogenase | ||
}} | }} | ||
Latest revision as of 17:45, 5 October 2023
| Tseng W-W, Wei A-C (2022) Kinetic mathematical modeling of oxidative phosphorylation in cardiomyocyte mitochondria. Cells 11:4020. https://doi.org/10.3390/cells11244020 |
Tseng W-W, Wei A-C (2022) Cells
Abstract: Oxidative phosphorylation (OXPHOS) is an oxygen-dependent process that consumes catabolized nutrients to produce adenosine triphosphate (ATP) to drive energy-dependent biological processes such as excitation-contraction coupling in cardiomyocytes. In addition to in vivo and in vitro experiments, in silico models are valuable for investigating the underlying mechanisms of OXPHOS and predicting its consequences in both physiological and pathological conditions. Here, we compare several prominent kinetic models of OXPHOS in cardiomyocytes. We examine how their mathematical expressions were derived, how their parameters were obtained, the conditions of their experimental counterparts, and the predictions they generated. We aim to explore the general landscape of energy production mechanisms in cardiomyocytes for future in silico models.
• Bioblast editor: Gnaiger E
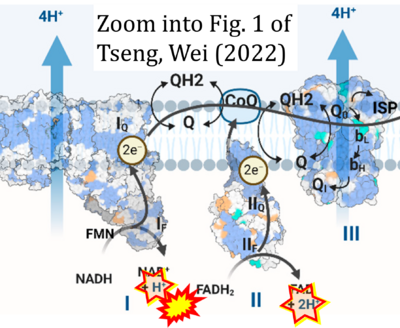
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase