Talk:MiPNet17.02 PBI-Shredder manual
Introduction
Application of high-resolution respirometry with gently perpared tissue homogenates offers a versatile tool to study mitochondrial function in small amounts of tissues. The PBI-Shredder SG3 (Fig. 1) is a low shear mechanical homogenization system, designed to apply reproducible force to the tissue with three positions of the force setting lever. This yields standardized, rapid and safe disruption of cells with preservation of intact, functional mitochondria. The laboratory-specific or even operator-specific protocols for tissue homogenization are thus standardized, providing reproducible and more consistent results for quantitative and inter-laboratory comparison. The easy handling enables especially beginners to obtain reliable results.
The PBI-Shredder HRR-Set includes standard Shredder-Tubes for ambient pressure processing, without and with a metal insert to disrupt tough cellular structures. In our primary applications with mouse and fish myocard and liver, Shredder-Tubes with and without metal inserts gave comparable results. Optimization of homogenization with various tissues will be possible using either type of Shredder-Tubes, force settings, and duration of shredding.
Materials and Chemicals
Components of the PBI-Shredder HRR-Set
Other materials
- Microbalance Mettler-Toledo, 0.01 mg display; Microbalance-Set
- Petri dish and 12-well tissue culture plate
- 15 ml Falcon tubes (1 per Shredder-Tube)
- 500 µl pipette with tips
- Filter paper or soft tissues
- Timer (1-60 s)
- Ice
Media
- BIOPS: The relaxing and organ preservation solution BIOPS contains 10 mM Ca-EGTA buffer, 0.1 µM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM K-MES, 0.5 mM DTT, 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phosphocreatine, pH 7.1 (MiPNet03.02). BIOPS can be stored frozen at -20°C.
- MiR06 or MiR07 (MiPNet14.13)
Sample preparation
Organ harvest
Heart and liver are excised from the sacrificed animal and immediately separated into specific subsamples and added into Falcon tubes containing sufficient ice-cold BIOPS to cover the entire tissue sample (30 ml for the entire mouse heart, trout heart or trout liver). Keep on ice and minimize transportation and storage time as far as possible.
Tissue preparation
Place the tissue sample into a small Petri dish with fresh ice-cold BIOPS on a cooling plate. The tissue should be covered with liquid.
Heart: Open the left ventricle of the heart by using a pair of small sharp scissors and a pair of sharp forceps. Cut out muscle tissue and omit pericardium. Place small muscle pieces into a 12-well plate with ice-cold respiration medium (MiR06/MiR07).
Determination of wet weight, Ww
Prepare tissue samples of about 4 mg Ww for mouse heart muscle and about 16 mg Ww of trout heart muscle or trout liver for two O2k-Chambers (half the Ww if one Shredder Tube should be used for one O2k-Chamber).
Move the samples with the pair of sharp forceps onto a filter paper. During this time of a few seconds, wipe off any liquid from the tip of the forceps with another filter paper. Then take the samples from the filter paper and touch it once more shortly onto a dry area of filter paper while holding it with the forceps. Afterwards, immediately place the samples onto a small plastic plate on the tared microbalance.
Immediately after reading the wet weight, the samples are cut into smaller pieces which are then transferred to the narrow Ram side of the Shredder-Tube using a pair of sharp forceps.
Tissue homogenization (shredding)
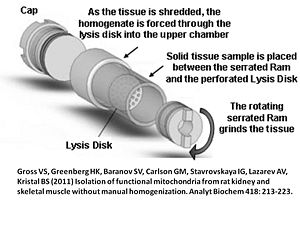
After evenly distributing the small tissue pieces on the Lysis Disk at the narrow Ram side of the Shredder-Tube, a serrated Shredder-Ram is inserted with a twisting motion to press the sample between the serrated surface and the Lysis Disk by using the Shredder-Tube Cap Tool (Figure 2).
500 µl Respiration Medium (e.g. MiR06) are then added to the Cap side (wide) of the tube. The total volume of sample and buffer during shredding should not exceed 0.7 to 0.8 ml (this prevents buffer from being forced into the threads of the cap where it might be lost during uncapping). The Shredder Tube is then capped with a Shredder-Screw Cap using the Shredder-Tube Cap Tool again (Figure 3).
Place the filled Shredder Tube into the pre-chilled Shredder Base, Ram side down, and twist to set the Ram into the holder in the Shredder Base. When the tube is seated securely, place the SG3 Driver onto the Cap, and briefly turn the driver on in order to seat the Driver bit into the crenellations of the Cap.
While pressing down the Driver with one hand, set the lever into the appropriate position for the sample. For mouse and trout heart as well as trout liver, 10 seconds at position 1 (weakest) followed by 5 seconds at position 2 (stronger) was evaluated as optimum regime, with a maximum of the sample passing through the Lysis Disk into the upper chamber of the Shredder-Tube, containing functionally intact mitochondria. Position 3 (strongest) was not required in these samples. This short processing time does not significantly heat the sample. It is recommended to use a timer for application of the shredder.
Removing the homogenate
To remove the processed homogenate, use the Shredder-Tube Cap Tool to remove the Shredder Cap from the Shredder Tube by anticlockwise rotation. Transfer the sample into a 15 ml Falcon on ice using a 500 µl pipette. To recover any residual sample, rinse the tube with fresh cold respiration medium and add to the homogenate. If there is any residual tissue under the Lysis Disk, try to rinse it out by pipetting thoroughly. Rinse with 4.5 ml in total. At the end there should be 5 ml of homogenate in the Falcon tube on ice. This volume is intended for use with two O2k-Chambers. Keep the sample on ice until used for HRR.
The homogenate obtained by this method may contain some tissue particles that are not homogenized, but complete cell permeabilization was obtained as evaluated by HRR. In this case, the only problem is the equal distribution of the homogenate into different O2k-Chambers.
Advantages and disadvantages
Advantages
- Standardized tissue preparations for obtaining disrupted cells with functional mitochondria.
- Highly reproducible results.
- Easy handling, especially for beginners.
- Minimum processing time of 10 min.
- Closed Shredder-Tubes ensure safety throughout the entire sample preparation process.
- Either the homogenate may be used directly for HRR, or the homogenization process may be followed by further isolation of mitochondria.
- The homogenate is suitable for optical measurements (e.g. O2k-Fluorometry with safranin for detection of mt-membrane potential) where a homogenous suspension is required.
Disadvantages
- A fraction of mitochondria is lost (c. 50% in our preparations), therefore about twice the amount of tissue is required compared to permeabilized fibres or homogenization of the total tissue with a potter.
- Since not all mitochondria are obtained from the tissue, tissue mass-specific mitochondrial respiratory capacity can be measured only on the basis of additional measurements of a mitochondrial marker (e.g. CS activity) in the total tissue and in the homogenate, to quantify the mt-yield and refer respiration of the homogenate to Ww of tissue.
- Since not all mitochondria are obtained from the tissue, it is difficult to evaluate if specific mitochondrial types are enriched or a representative subsample of all mitochondria is obtained.
- The cytochrome c effect is larger in homogenate compared to permeabilized fibres, hence a small degree of functional impairment of mitochondria is caused by the homogenization process.
Acknowledgements
Contribution to K-Regio project MitoCom Tyrol, funded in part by the Tyrolian Government and the European Regional Development Fund.
References
- Gross VS, Greenberg HK, Baranov SV, Carlson GM, Stavrovskaya IG, Lazarev AV, Kristal BS (2011) Isolation of functional mitochondria from rat kidney and skeletal muscle without manual homogenization. Analyt Biochem 418: 213-223.
- Doerrier C, Draxl A, Wiethüchter A, Eigentler A, Gnaiger E (2012) Mitochondrial respiration in permeabilized fibres versus homogenate from mouse myocardium. An application study with the PBI-Shredder. Mitocondr Physol Network 17.03: 1-12.
- Pressure BioSciences Inc. The Shredder SG3 and Shredder PULSE Tubes: Product Specification Sheet: 1-2.
- Pressure BioSciences Inc. The Shredder SG3: User Manual: 1-16.
Mitochondr Physiol Network – MiPNet Manuals and Protocols
- Selected media and chemicals for respirometry with mitochondria and permeabilized cells. MiPNet03.02: 1-9.
- Mitochondrial respiration medium - MiR06. MiPNet14.13: 1-4.
Author contributions and progress
Prepared by Draxl A, Eigentler A and Gnaiger E in February 2012. DA performed the experiments.
- First on-line version: 2012-02-27.