Sharma 2021 Int J Mol Sci: Difference between revisions
(Created page with "{{Publication |title=Sharma C, Kim S, Nam Y, Jung UJ, Kim SR (2021) Mitochondrial dysfunction as a driver of cognitive impairment in Alzheimer's disease. Int J Mol Sci 22:4850...") |
No edit summary |
||
| Line 8: | Line 8: | ||
|editor=Gnaiger E | |editor=Gnaiger E | ||
}} | }} | ||
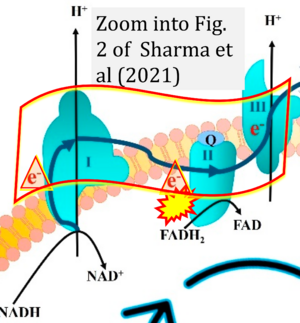
[[File:Sharma 2021 Int J Mol Sci CORRECTION.png|right|300px]] | [[File:Sharma 2021 Int J Mol Sci CORRECTION.png|right|300px]] | ||
{{Template:Correction FADH2 and S-pathway}} | {{Template:Correction FADH2 and S-pathway}} | ||
Revision as of 18:20, 13 October 2023
| Sharma C, Kim S, Nam Y, Jung UJ, Kim SR (2021) Mitochondrial dysfunction as a driver of cognitive impairment in Alzheimer's disease. Int J Mol Sci 22:4850. https://doi.org/10.3390/ijms22094850 |
Sharma C, Kim S, Nam Y, Jung UJ, Kim SR (2021) Int J Mol Sci
Abstract: Alzheimer's disease (AD) is the most frequent cause of age-related neurodegeneration and cognitive impairment, and there are currently no broadly effective therapies. The underlying pathogenesis is complex, but a growing body of evidence implicates mitochondrial dysfunction as a common pathomechanism involved in many of the hallmark features of the AD brain, such as formation of amyloid-beta (Aβ) aggregates (amyloid plaques), neurofibrillary tangles, cholinergic system dysfunction, impaired synaptic transmission and plasticity, oxidative stress, and neuroinflammation, that lead to neurodegeneration and cognitive dysfunction. Indeed, mitochondrial dysfunction concomitant with progressive accumulation of mitochondrial Aβ is an early event in AD pathogenesis. Healthy mitochondria are critical for providing sufficient energy to maintain endogenous neuroprotective and reparative mechanisms, while disturbances in mitochondrial function, motility, fission, and fusion lead to neuronal malfunction and degeneration associated with excess free radical production and reduced intracellular calcium buffering. In addition, mitochondrial dysfunction can contribute to amyloid-β precursor protein (APP) expression and misprocessing to produce pathogenic fragments (e.g., Aβ1-40). Given this background, we present an overview of the importance of mitochondria for maintenance of neuronal function and how mitochondrial dysfunction acts as a driver of cognitive impairment in AD. Additionally, we provide a brief summary of possible treatments targeting mitochondrial dysfunction as therapeutic approaches for AD.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«