Difference between revisions of "PM-pathway control state"
From Bioblast
Beno Marija (talk | contribs) |
|||
| Line 30: | Line 30: | ||
::::* '''''P - E''''' | ::::* '''''P - E''''' | ||
:::: [[CCCP]] is titrated stepwise to maximum flux, to evaluate limitation of OXPHOS by the phosphorylation system, expressed as the apparent [[excess E-P capacity factor |excess ''E-P'' capacity factor]] (''E-P'' coupling control factor), ''j<sub>ExP</sub>'' = (''E-P'')/''E'' = 1-''P/E''. If ''j<sub>ExP</sub>''>0, then the [[ | :::: [[CCCP]] is titrated stepwise to maximum flux, to evaluate limitation of OXPHOS by the phosphorylation system, expressed as the apparent [[excess E-P capacity factor |excess ''E-P'' capacity factor]] (''E-P'' coupling control factor), ''j<sub>ExP</sub>'' = (''E-P'')/''E'' = 1-''P/E''. If ''j<sub>ExP</sub>''>0, then the [[ET-pathway coupling efficiency]] rather than the [[OXPHOS coupling efficiency]] is the proper expression of coupling, ''j<sub>≈E</sub>'' = ''≈E/E'' = (''E-L'')/''E'' = 1-''L/E''. | ||
== Discussion == | == Discussion == | ||
:::: The [[Pyruvate anaplerotic pathway control state]] (pyruvate alone) is not an | :::: The [[Pyruvate anaplerotic pathway control state]] (pyruvate alone) is not an ET-pathway competent substrate state in most mt-preparations, since acetyl-CoA accumulates without the co-substrate (oxaloacetate) of citrate synthase. | ||
:::: The [[Malate anaplerotic pathway control state]] (M alone) is not an | :::: The [[Malate anaplerotic pathway control state]] (M alone) is not an ET-pathway competent substrate state in many mt-preparations, since oxaloacetate accumulates without the co-substrate (acetyl-CoA) of citrate synthase. | ||
Revision as of 13:42, 20 October 2017
Description
MitoPathway control state: N
SUIT protocol: SUIT_FNSGp(PGM)01 - SUIT_RP1
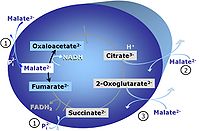
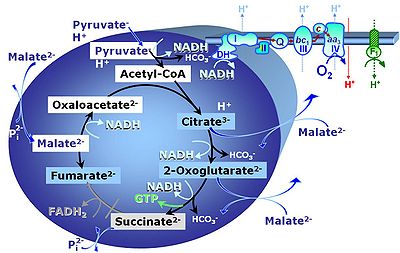
Pyruvate (P) is oxidatively decarboxylated to acetyl-CoA and CO2, yielding NADH catalyzed by pyruvate dehydrogenase. Malate (M) is oxidized to oxaloacetate by mt-malate dehydrogenase located in the mitochondrial matrix. Condensation of oxaloacate with acetyl-CoA yields citrate (citrate synthase). 2-oxoglutarate (α-ketoglutarate) is formed from isocitrate (isocitrate dehydrogenase).
Abbreviation: PM
Reference: Gnaiger 2014 MitoPathways - Chapter 3.2
MitoPedia concepts:
SUIT state
PML
- SUIT_FNSGp(PGM)01: 1PM 2D&Dc (&DcNADH) 3U 4Oct 5G 6S 7Rot 8Gp 9Ama 10Tm 11Azd
PMP
- SUIT_FNSGp(PGM)01: 1PM 2D&Dc (&DcNADH) 3U 4Oct 5G 6S 7Rot 8Gp 9Ama 10Tm 11Azd
PME
- SUIT_FNSGp(PGM)01: 1PM 2D 2c (2NADH) 3U 4Oct 5G 6S 7Rot 8Gp 9Ama 10Tm
Linear coupling control in the N-pathway control state: L – P - E
- L - P
- OXPHOS coupling efficiency (P-L or ≈P control factor), j≈P = ≈P/P = (P-L)/P = 1-L/P, is measured in the CI-linked substrate state, with defined coupling sites (CI, CIII, CIV) and at high flux.
- P - E
- CCCP is titrated stepwise to maximum flux, to evaluate limitation of OXPHOS by the phosphorylation system, expressed as the apparent excess E-P capacity factor (E-P coupling control factor), jExP = (E-P)/E = 1-P/E. If jExP>0, then the ET-pathway coupling efficiency rather than the OXPHOS coupling efficiency is the proper expression of coupling, j≈E = ≈E/E = (E-L)/E = 1-L/E.
Discussion
- The Pyruvate anaplerotic pathway control state (pyruvate alone) is not an ET-pathway competent substrate state in most mt-preparations, since acetyl-CoA accumulates without the co-substrate (oxaloacetate) of citrate synthase.
- The Malate anaplerotic pathway control state (M alone) is not an ET-pathway competent substrate state in many mt-preparations, since oxaloacetate accumulates without the co-substrate (acetyl-CoA) of citrate synthase.