Kimoloi 2019 MiPschool Coimbra: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 6: | Line 6: | ||
|event=MiPschool Coimbra 2019 | |event=MiPschool Coimbra 2019 | ||
|abstract=[[Image:MITOEAGLE-logo.jpg|left|100px|link=http://www.mitoeagle.org/index.php/MitoEAGLE|COST Action MitoEAGLE]] | |abstract=[[Image:MITOEAGLE-logo.jpg|left|100px|link=http://www.mitoeagle.org/index.php/MitoEAGLE|COST Action MitoEAGLE]] | ||
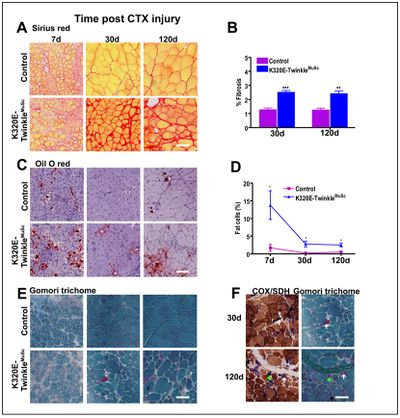
Muscle satellite cells (MuScs) play an important role in the regeneration of skeletal muscle during the postnatal life [1]. However, their regenerative efficiency declines with aging, a phenomenon that might be due to both intrinsic and extrinsic factors [2, 3]. One of the putative intrinsic factors might be loss of mtDNA integrity-induced mitochondrial dysfunction. We therefore generated a mouse strain which expresses, upon Pax7-driven Cre-recombination (Rosa26-Stop-construct), a dominant-negative mutant of the mitochondrial helicase Twinkle [4], thus strongly enhancing the generation of ΔmtDNA molecules in MuSCs. Tibialis anterior muscles of these mice and age-matched controls were then injured with cardiotoxin and the regeneration efficiency analysed at different time points post- injury. Histochemical analysis revealed markedly impaired regeneration as demonstrated by altered tissue architecture, increased collagen fibrosis, fiber atrophy as well as immune cells and adipocyte infiltration. These findings indicate that ΔmtDNA-induced mitochondrial dysfunction is indeed an important factor that impairs MuSCs regenerative efficiency. Targeted therapeutic strategies to maintain MuSCs mtDNA integrity and mitochondrial function, might thus potentially sustain regenerative capacity of skeletal muscle. The generated mouse model is a valuable model to test such strategies in future. | Muscle satellite cells (MuScs) play an important role in the regeneration of skeletal muscle during the postnatal life [1]. However, their regenerative efficiency declines with aging, a phenomenon that might be due to both intrinsic and extrinsic factors [2,3]. One of the putative intrinsic factors might be loss of mtDNA integrity-induced mitochondrial dysfunction. We therefore generated a mouse strain which expresses, upon Pax7-driven Cre-recombination (Rosa26-Stop-construct), a dominant-negative mutant of the mitochondrial helicase Twinkle [4], thus strongly enhancing the generation of ΔmtDNA molecules in MuSCs. Tibialis anterior muscles of these mice and age-matched controls were then injured with cardiotoxin and the regeneration efficiency analysed at different time points post- injury. Histochemical analysis revealed markedly impaired regeneration as demonstrated by altered tissue architecture, increased collagen fibrosis, fiber atrophy as well as immune cells and adipocyte infiltration. These findings indicate that ΔmtDNA-induced mitochondrial dysfunction is indeed an important factor that impairs MuSCs regenerative efficiency. Targeted therapeutic strategies to maintain MuSCs mtDNA integrity and mitochondrial function, might thus potentially sustain regenerative capacity of skeletal muscle. The generated mouse model is a valuable model to test such strategies in future. | ||
|keywords=Muscle ageing, Regeneration, Mitochondria, Muscle stem cells-fibrosis, | |keywords=Muscle ageing, Regeneration, Mitochondria, Muscle stem cells-fibrosis, | ||
|editor=[[Plangger M]], | |editor=[[Plangger M]], | ||
}} | }} | ||
{{Labeling}} | {{Labeling | ||
|area=mtDNA;mt-genetics | |||
|diseases=Aging;senescence | |||
|organism=Mouse | |||
|tissues=Stem cells | |||
}} | |||
== Affiliations == | == Affiliations == | ||
::::Kimoloi S(1), Baris OR(1), Wiesner RJ(1,2,3) | ::::Kimoloi S(1), Baris OR(1), Wiesner RJ(1,2,3) | ||
| Line 42: | Line 47: | ||
== References == | == References == | ||
::::#Mauro | ::::#Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493-5. | ||
::::#Brack, | ::::#Brack AS, Munoz-Canoves P (2016) The ins and outs of muscle stem cell aging. Skelet Muscle 6:1. | ||
::::#Sousa-Victor, | ::::#Sousa-Victor P, García-Prat L, Serrano AL, Perdiguero E, Muñoz-Cánoves P (2015) Muscle stem cell aging: regulation and rejuvenation. Trends Endocrinol Metab 26:287-96. | ||
::::#Baris, | ::::#Baris OR, Ederer S, Neuhaus JF, von Kleist-Retzow JC, Wunderlich CM, Pal M, Wunderlich FT, Peeva V, Zsurka G, Kunz WS, Hickethier T, Bunck AC, Stöckigt F, Schrickel JW, Wiesner RJ (2015) Mosaic Deficiency in Mitochondrial Oxidative Metabolism Promotes Cardiac Arrhythmia during Aging. Cell Metab 21:667-77. | ||
Latest revision as of 15:42, 27 June 2019
| Mitochondrial DNA (mtDNA) maintenance defects impairs muscle stem cells regenerative efficiency. |
Link: MitoEAGLE
Kimoloi S, Baris OR, Wiesner RJ (2019)
Event: MiPschool Coimbra 2019
Muscle satellite cells (MuScs) play an important role in the regeneration of skeletal muscle during the postnatal life [1]. However, their regenerative efficiency declines with aging, a phenomenon that might be due to both intrinsic and extrinsic factors [2,3]. One of the putative intrinsic factors might be loss of mtDNA integrity-induced mitochondrial dysfunction. We therefore generated a mouse strain which expresses, upon Pax7-driven Cre-recombination (Rosa26-Stop-construct), a dominant-negative mutant of the mitochondrial helicase Twinkle [4], thus strongly enhancing the generation of ΔmtDNA molecules in MuSCs. Tibialis anterior muscles of these mice and age-matched controls were then injured with cardiotoxin and the regeneration efficiency analysed at different time points post- injury. Histochemical analysis revealed markedly impaired regeneration as demonstrated by altered tissue architecture, increased collagen fibrosis, fiber atrophy as well as immune cells and adipocyte infiltration. These findings indicate that ΔmtDNA-induced mitochondrial dysfunction is indeed an important factor that impairs MuSCs regenerative efficiency. Targeted therapeutic strategies to maintain MuSCs mtDNA integrity and mitochondrial function, might thus potentially sustain regenerative capacity of skeletal muscle. The generated mouse model is a valuable model to test such strategies in future.
• Keywords: Muscle ageing, Regeneration, Mitochondria, Muscle stem cells-fibrosis • Bioblast editor: Plangger M
Labels: MiParea: mtDNA;mt-genetics Pathology: Aging;senescence
Organism: Mouse Tissue;cell: Stem cells
Affiliations
- Kimoloi S(1), Baris OR(1), Wiesner RJ(1,2,3)
- Center Physiology Pathophysiology, Inst Vegetative Physiology, Medical Fac
- Center Molecular Medicine Cologne, CMMC; Univ Köln
- Cologne Excellence Cluster Cellular Stress Responses Ageing-associated Diseases (CECAD); Köln, Germany
- Kimoloi S(1), Baris OR(1), Wiesner RJ(1,2,3)
Figures
Figure 1: Mitochondrial DNA maintainance defecfects in MuSc results in fibrosis, fat infiltration and ragged red phenotype in regenerated muscles. A and B) Sirius red staining analysis showing increased fibrosis at 30 and 120d post CTX injury, C and D) oil O red showing increased adipocyte infiltration in mutant mice at 30 and 120d post CTX injury, E) modified Gomori trichrome staining indicating presence of RRFs in mutant mice, F) serial sections stained for COX-SDH and Gomori trichrome. **p<0.01 t-test *p<0.05 two-way ANOVA, Scale bar=100µm.
References
- Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493-5.
- Brack AS, Munoz-Canoves P (2016) The ins and outs of muscle stem cell aging. Skelet Muscle 6:1.
- Sousa-Victor P, García-Prat L, Serrano AL, Perdiguero E, Muñoz-Cánoves P (2015) Muscle stem cell aging: regulation and rejuvenation. Trends Endocrinol Metab 26:287-96.
- Baris OR, Ederer S, Neuhaus JF, von Kleist-Retzow JC, Wunderlich CM, Pal M, Wunderlich FT, Peeva V, Zsurka G, Kunz WS, Hickethier T, Bunck AC, Stöckigt F, Schrickel JW, Wiesner RJ (2015) Mosaic Deficiency in Mitochondrial Oxidative Metabolism Promotes Cardiac Arrhythmia during Aging. Cell Metab 21:667-77.