Kim 2010 Korean Diabetes J
| Kim EH, Koh EH, Park JY, Lee KU (2010) Adenine nucleotide translocator as a regulator of mitochondrial function: implication in the pathogenesis of metabolic syndrome. Korean Diabetes J 34:146-53. https://doi.org/10.4093/kdj.2010.34.3.146 |
Kim EH, Koh EH, Park JY, Lee KU (2010) Korean Diabetes J
Abstract: Mitochondria play key roles in energy production and intracellular reactive oxygen species (ROS) generation. Lines of evidence have shown that mitochondrial dysfunction contributes to the development of metabolic syndrome. The causes of mitochondrial dysfunction are complex, but overnutrition and sedentary living are among the best known causes of mitochondrial dysfunction. ATP synthesized in the mitochondria is exchanged for cytosolic ADP by adenine nucleotide translocator (ANT) to provide a continuous supply of ADP to mitochondria. We recently found that ANT function is essential for peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1alpha)'s action on endothelial cells. PGC-1alpha is a transcriptional coactivator of nuclear receptors, playing an important role in fatty acid oxidation and mitochondrial biogenesis. Recent studies have shown that PGC-1alpha decreases intracellular ROS generation by increasing the expression of antioxidant genes. In our study, PGC-1alpha reduced cell apoptosis and ROS generation in endothelial cells by increasing ATP/ADP translocase activity of ANT and ANT1 expression. Here we review the role of ANT in maintaining proper mitochondrial function, and possible role of ANT dysfunction in the pathogenesis of metabolic syndrome.
• Bioblast editor: Gnaiger E
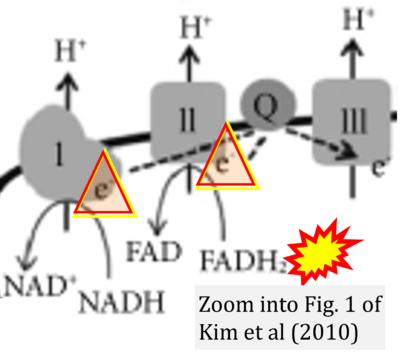
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase