Ishii 2012 Front Oncol
| Ishii I, Harada Y, Kasahara T (2012) Reprofiling a classical anthelmintic, pyrvinium pamoate, as an anti-cancer drug targeting mitochondrial respiration. Front Oncol 2:137. https://doi.org/10.3389/fonc.2012.00137 |
Ishii I, Harada Y, Kasahara T (2012) Front Oncol
Abstract: Pyrvinium pamoate (PP) is an FDA-approved classical anthelmintic, but is now attracting particular attention as an anti-cancer drug after recent findings of its potent cytotoxicity against various cancer cell lines only during glucose starvation, as well as its anti-tumor activity against hypovascular pancreatic cancer cells transplanted in mice. The molecular mechanisms by which PP promotes such preferential toxicity against cancer cells are currently under extensive investigation. PP suppressed the NADH-fumarate reductase system that mediates a reverse reaction of the mitochondrial electron-transport chain complex II in anaerobic organisms such as parasitic helminthes or mammalian cells under tumor microenvironment-mimicking hypoglycemic/hypoxic conditions, thereby inhibiting efficient ATP production. PP also inhibited the unfolded protein response induced by glucose starvation, thereby inhibiting the proliferation of pancreatic cancer cells. Even under normoglycemic/normoxic conditions, PP suppressed the mitochondrial electron-transport chain complex I and thereby STAT3, inhibiting the proliferation of myeloma/erythroleukemia cells. Here, we review accumulating knowledge on its working mechanisms and evaluate PP as a novel anti-cancer drug that targets mitochondrial respiration.
• Bioblast editor: Gnaiger E
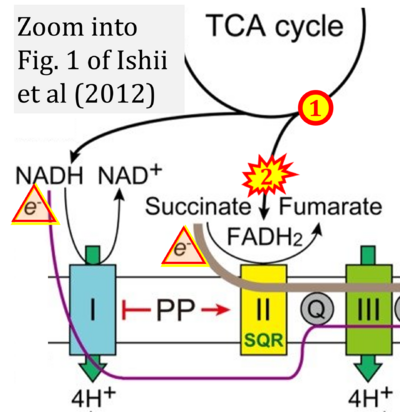
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase