Goncalves Debora 2019 MiPschool Coimbra
| Aging effects on mitochondrial control factors in Pink1 knockout Drosophila melanogaster. |
Link: MitoEAGLE
Goncalves DF, Courtes AA, Hartmann DD, Carvalho PR, Marques DM, Machado ML, Furtado AV, Soares FA, Dalla Corte CL (2019)
Event: MiPschool Coimbra 2019
Parkinson disease (PD) is considered the second most common neurodegenerative disorder in the world and is related mainly with aging. PD is characterized by loss of dopaminergic neurons and formation of protein aggregates, such as α-synuclein [1], leading to motor dysfunction, and impairment of cognitive and memory functions [2]. The pathology and symptoms of PD are well described, although its mechanisms and causes remain unclear. One mechanism involved in PD is mitochondrial dysfunction [3]. Mutation in genes involved in mitochondrial quality control, such as PARK2 and Pink1, produce PD symptoms [4]. These genes code for proteins such as PINK1 (PTEN induced kinase 1) that is a serine/threonine kinase involved in mitochondrial network quality control [5]. In this work we evaluated mitochondrial quality control using Pink1 knockout of Drosophila melanogaster in different life time.
The analyses were performed on O2k-system high-resolution oxygraphy (Oroboros Instruments, Innsbruck, Austria). Two male flies were previous homogenized in respiration medium-MIR05 (0.5mM EGTA, 3mM MgCl2, 60mM lactobionic acid, 20mM taurine, 10mM KH2PO4, 20mM HEPES, 110mM sucrose, 0.1 mg/mL fatty acid free BSA) and added to the oroboros chamber containing MIR05 at 37 °C. The protocol consisted of a sequential titration of multiple substrates, uncouplers and inhibitors (SUIT protocol) [6]. After signal stabilization, the experimental SUIT protocol was performed by sequential addition of pyruvate (5 mM), malate (2 mM) and glutamate (10 mM); ADP (5 mM); succinate (10 mM); oligomycin (2.5 μM); carbonyl cyanide-4-(tri-fluoromethoxy) phenylhydrazone (FCCP -titrations of 0.25 μM until reaching the maximum oxygen consumption); rotenone (0.5 μM); malonate (5 mM) and antimycin (2.5 μM). In order to evaluate the mitochondrial quality control, mitochondrial control factors were calculated;OXPHOS coupling efficiency, ETS coupling efficiency, respiratory control ratio and excess capacity factor. We consider each assay as one experimental replicate, our results are presented as a media of 5 different assays. Statistical analyses were performed using t-test to demonstrate significant difference between control flies and Pink1 knockout flies.
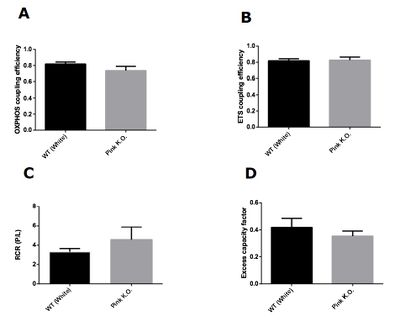
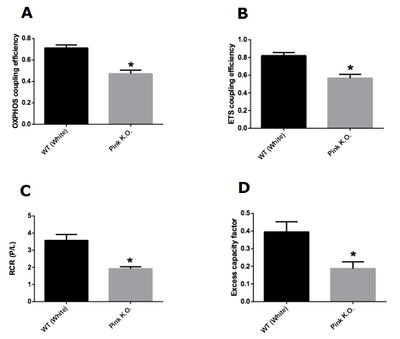
Our results demonstrate that knockout flies of Pink1 with 3 days of life did not present difference in any mitochondrial control factors evaluated. On the other hand, knockout flies of Pink1 with 15 days had a significant decrease in all mitochondrial control factors evaluated. Taken together, our results showed that damage in mitochondrial network caused by Pink1 deletion is more harmful with aging. The Pink1 gene is responsible for codify a serine threonine kinase (PINK1) responsible for maintaining mitochondrial quality control trough the autophagy process. In depolarized mitochondria, there is an accumulation of PINK1 on mitochondrial membranes, resulting in a recruitment of Parkin which is a ubiquitin ligase normally located on cytosol and involved in autophagosome formation [7]. The formation of phagosome starts the mitophagy process that is defined as selective removal of damaged mitochondria from mitochondrial network. With our results we demonstrate that accumulated damage is capable to change mitochondrial control factors, however, young knockout Pink1 flies did not present alterations in mitochondrial factors, apparently maintaining the quality of mitochondrial network even with the loss of PINK1 effects.
• Bioblast editor: Plangger M
Labels: MiParea: Respiration, Genetic knockout;overexpression Pathology: Aging;senescence, Parkinson's
Organism: Drosophila
Preparation: Homogenate
Regulation: Coupling efficiency;uncoupling Coupling state: LEAK, OXPHOS, ET Pathway: N, S, NS, ROX HRR: Oxygraph-2k
Affiliations
- Gonçalves DF(1), Courtes AA, Hartmann DD(1), Carvalho PR(1), Marques DM(1), Machado ML(1), Furtado AV(1), Soares FA(1), Dalla Corte CL(1,2)
- Biochemistry and molecular biology Dpt, Univ Federal Santa Maria
- Univ Federal Pampa; Brazil. - [email protected]
- Gonçalves DF(1), Courtes AA, Hartmann DD(1), Carvalho PR(1), Marques DM(1), Machado ML(1), Furtado AV(1), Soares FA(1), Dalla Corte CL(1,2)
Figures
Figure 1: Mitochondrial control factors in Pink1 knockout flies with 3 days of age. (A) OXPHOS coupling efficiency (1-L/P). (B) ETS coupling efficiency (1-(L/E)). (C) Respiratory control ratio (P/L). (D) Excess capacity factor (1-(P/E)). Data are reported as mean ± S.E.M., n=5.
Figure 2: Mitochondrial control factors in Pink1 knockout flies with 15 days of age. (A) OXPHOS coupling efficiency (1-L/P). (B) ETS coupling efficiency (1-(L/E)). (C) Respiratory control ratio (P/L). (D) Excess capacity factor (1-(P/E)). Data are reported as mean ± S.E.M., n=5. *Indicates p < 0.05 as compared to the control group
References
- Requejo-Aguilar R, Lopez-Fabuel I, Fernandez E, Martins LM, Almeida A, Bolaños JP(2014) PINK1 deficiency sustains cell proliferation by reprogramming glucose metabolism through HIF1. Nat Commun 5:4514.
- Goetz CG (2011) The history of Parkinson’s disease: early clinical descriptions and neurological therapies. Cold Spring Harb Perspect Med 1:a008862.
- Ammal Kaidery N, Thomas B (2018) Current perspective of mitochondrial biology in Parkinson’s disease. Neurochem Int 117:91-113.
- Wang HL, Chou AH, Wu AS, Chen SY, Weng YH, Kao YC, Yeh TH, Chu PJ, Lu CS (2011) PARK6 PINK1 mutants are defective in maintaining mitochondrial membrane potential and inhibiting ROS formation of substantia nigra dopaminergic neurons. Biochim Biophys Acta 1812:674–84.
- Gautier CA, Kitada T, Shen J (2008) Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci 105:11364–69.
- Pesta D, Gnaiger E (2012) High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25-58.
- Requejo-Aguilar R, Bolaños JP (2016) Mitochondrial control of cell bioenergetics in Parkinson’s disease. Free Radic Biol Med 100:123–37.