Faraday constant: Difference between revisions
From Bioblast
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
Communicated by [[Gnaiger E]] 2018-10-18 | Communicated by [[Gnaiger E]] 2018-10-18 | ||
[[File:Table Physical constants.png|left|400px|thumb|]] | [[File:Table Physical constants.png|left|400px|thumb|]] | ||
== The 'faraday' as a unit of charge == | |||
:::: The faraday can be considered as a unit of charge (1 faraday of charge per mole) based on the Faraday constant. | |||
== References == | == References == | ||
::::# Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - [https://www.bipm.org/utils/common/pdf/si-brochure/SI-Brochure-9-EN.pdf »Open Access pdf«] | ::::# Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - [https://www.bipm.org/utils/common/pdf/si-brochure/SI-Brochure-9-EN.pdf »Open Access pdf«] | ||
::::# Gnaiger E (2019) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Mitochondr Physiol Network 24.05. Oroboros MiPNet Publications, Innsbruck:112 pp. - [[Gnaiger 2019 MitoPathways |»Bioblast link«]] | ::::# Gnaiger E (2019) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Mitochondr Physiol Network 24.05. Oroboros MiPNet Publications, Innsbruck:112 pp. - [[Gnaiger 2019 MitoPathways |»Bioblast link«]] | ||
<br /> | |||
{{Keywords SI base units}} | |||
{{MitoPedia concepts | {{MitoPedia concepts | ||
|mitopedia concept=Ergodynamics | |mitopedia concept=Ergodynamics | ||
}} | }} | ||
Revision as of 10:42, 5 September 2019
Description
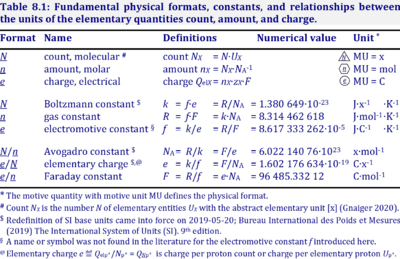
The Faraday constant, F, links the electric charge [C] to amount [mol], and thus relates the electrical format, e [C], to the molar format, n [mol]. The Farady constant, F = e·NA = 96,485.33 C/mol, is the product of elementary charge, e = 1.602176634∙10-19 C/x, and the Avogadro constant, NA = 6.02214076∙1023 x/mol. The dimensionless unit [x] is not explicitely considered by IUPAC.
Abbreviation: F [C/mol]
Reference: https://www.bipm.org/en/measurement-units/rev-si/
Communicated by Gnaiger E 2018-10-18
The 'faraday' as a unit of charge
- The faraday can be considered as a unit of charge (1 faraday of charge per mole) based on the Faraday constant.
References
- Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - »Open Access pdf«
- Gnaiger E (2019) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Mitochondr Physiol Network 24.05. Oroboros MiPNet Publications, Innsbruck:112 pp. - »Bioblast link«
Template:Keywords SI base units
MitoPedia concepts:
Ergodynamics