Difference between revisions of "Chinopoulos 2012 Abstract Bioblast"
| (11 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Abstract | {{Abstract | ||
|title=Dobolyi A, Ostergaard E, | |title=Dobolyi A, Ostergaard E, Bago AG, Palkovits M, Adam-Vizi V, Chinopoulos C (2012) Exclusive neuronal expression of SUCLA2 in the human brain. Mitochondr Physiol Network 17.12. | ||
|info=[[MiPNet17.12 Bioblast 2012|MiPNet17.12 Bioblast 2012 - Open Access]] | |info=[[MiPNet17.12 Bioblast 2012|MiPNet17.12 Bioblast 2012 - Open Access]] | ||
|authors=Chinopoulos C | |authors=Chinopoulos C | ||
|year=2012 | |year=2012 | ||
|event=[[Bioblast 2012]] | |event=[[Bioblast 2012]] | ||
|abstract=SUCLA2 encodes for the ADP-forming | |abstract=[[File:Christos Chinopoulos.jpg|right|150px|Christos Chinopoulos]] | ||
CoA ligase, an enzyme of the citric acid cycle. Mutations in SUCLA2 lead | SUCLA2 encodes for the ADP-forming β-subunit (A-SUCL-β) of succinyl CoA ligase, an enzyme of the citric acid cycle [1]. Mutations in SUCLA2 lead to a mitochondrial disorder associated with mitochondrial DNA depletion [2]. This mitochondrial disorder manifests as neonatal encephalomyopathy exhibiting dystonia, deafness and pronounced lesions in the basal ganglia [3]. Despite that a SUCLA2 gene defect results in distinct brain pathology, precise localization of the encoded protein has never been investigated. Here we show that the immunoreactivity of A-SUCL-β in the human cerebral cortex was present exclusively in neurons, identified by their morphology and visualized by double labelling with a fluorescent Nissl dye. The A-SUCL-β immunoreactivity co-localized >99% with that of the d-subunit of the mitochondrial F<sub>0</sub>-F<sub>1</sub> ATP synthase. Specificity of the anti-A-SUCL-β antiserum was verified by the absence of labelling in fibroblasts from a patient with a complete deletion of SUCLA2. A-SUCL-β immunoreactivity was absent in glial cells, identified by antibodies directed against the glial markers GFAP and S100. Our work establishes that SUCLA2 is expressed exclusively in neurons in the human cerebral cortex (Fig. 1). Therefore, all encephalopathic features of the disease emerging by mutations in this gene originate solely from the neuronal cell population. | ||
to a mitochondrial disorder associated with mitochondrial DNA depletion. | # Lambeth DO, Tews KN, Adkins S, Frohlich D, Milavetz BI (2004) Expression of two succinyl-CoA synthetases with different nucleotide specificities in mammalian tissues. J Biol Chem 279: 36621-36624. | ||
This mitochondrial disorder manifests as neonatal encephalomyopathy | # Ostergaard E (2008) Disorders caused by deficiency of succinate-CoA ligase. J Inherit Metab Dis 31: 226-229. | ||
exhibiting dystonia, deafness and pronounced lesions in the basal | # Carrozzo R, Onisi-Vici C, Steuerwald U, Lucioli S, Deodato F, Di GS, Bertini E, Franke B, Kluijtmans LA, Meschini MC, Rizzo C, Piemonte F, Rodenburg R, Santer R, Santorelli FM, van RA, Vermunt-de KD, Morava E, Wevers RA (2007) SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain 130: 862-874. | ||
ganglia. Despite that a SUCLA2 gene defect results in distinct brain | |keywords=SUCLA2, Mitochondrial disorder, Cortex, | ||
pathology, precise localization of the encoded protein has never been | |mipnetlab=HU Budapest Chinopoulos C, | ||
investigated. Here we show that the immunoreactivity of A-SUCL- | |||
human cerebral cortex was present exclusively in neurons, identified by | |||
their morphology and visualized by double labelling with a fluorescent | |||
Nissl dye. The A-SUCL- | |||
the d-subunit of the mitochondrial | |||
the anti-A-SUCL- | |||
fibroblasts from a patient with a complete deletion of SUCLA2. A-SUCL- | |||
immunoreactivity was absent in glial cells, identified by antibodies | |||
directed against the glial markers GFAP and S100. Our work establishes | |||
that SUCLA2 is expressed exclusively in neurons in the human cerebral | |||
cortex. Therefore, all encephalopathic features of the disease emerging | |||
by mutations in this gene originate solely from the neuronal cell | |||
|keywords=SUCLA2, Mitochondrial disorder, Cortex, | |||
|mipnetlab=HU Budapest Chinopoulos C, | |||
|journal=Mitochondr Physiol Network | |journal=Mitochondr Physiol Network | ||
|articletype=Abstract | |articletype=Abstract | ||
}} | }} | ||
{{Labeling | {{Labeling | ||
|organism=Human | |organism=Human | ||
|tissues= | |tissues=Nervous system | ||
|injuries=Mitochondrial disease | |||
|journal=Mitochondr Physiol Network | |journal=Mitochondr Physiol Network | ||
|articletype=Abstract | |articletype=Abstract | ||
}} | }} | ||
__NOTOC__ | __NOTOC__ | ||
== Affiliations and author contributions == | == Affiliations and author contributions == | ||
| Line 53: | Line 37: | ||
(5) Department of Medical Biochemistry, Semmelweis University, Hungary | (5) Department of Medical Biochemistry, Semmelweis University, Hungary | ||
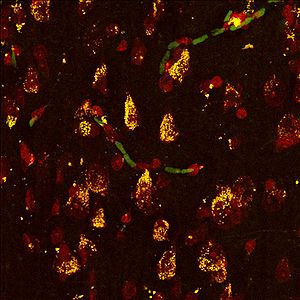
== Figure 1 == | |||
[[File:Christos Nissl Sucla2.jpg|300px|Nissl Sucla2]] | |||
Co-localization of fluorescent Nissl staining the cytosol of neurons (red) | |||
and the ADP-forming β-subunit (A-SUCL-β) | |||
of succinyl CoA ligase (green-->yellow) in human temporal cortex. | |||
== Help == | == Help == | ||
* [[MitoPedia: Terms and abbreviations]] | |||
* [[MitoPedia | |||
Latest revision as of 17:15, 3 February 2016
| Dobolyi A, Ostergaard E, Bago AG, Palkovits M, Adam-Vizi V, Chinopoulos C (2012) Exclusive neuronal expression of SUCLA2 in the human brain. Mitochondr Physiol Network 17.12. |
Link: MiPNet17.12 Bioblast 2012 - Open Access
Chinopoulos C (2012)
Event: Bioblast 2012
SUCLA2 encodes for the ADP-forming β-subunit (A-SUCL-β) of succinyl CoA ligase, an enzyme of the citric acid cycle [1]. Mutations in SUCLA2 lead to a mitochondrial disorder associated with mitochondrial DNA depletion [2]. This mitochondrial disorder manifests as neonatal encephalomyopathy exhibiting dystonia, deafness and pronounced lesions in the basal ganglia [3]. Despite that a SUCLA2 gene defect results in distinct brain pathology, precise localization of the encoded protein has never been investigated. Here we show that the immunoreactivity of A-SUCL-β in the human cerebral cortex was present exclusively in neurons, identified by their morphology and visualized by double labelling with a fluorescent Nissl dye. The A-SUCL-β immunoreactivity co-localized >99% with that of the d-subunit of the mitochondrial F0-F1 ATP synthase. Specificity of the anti-A-SUCL-β antiserum was verified by the absence of labelling in fibroblasts from a patient with a complete deletion of SUCLA2. A-SUCL-β immunoreactivity was absent in glial cells, identified by antibodies directed against the glial markers GFAP and S100. Our work establishes that SUCLA2 is expressed exclusively in neurons in the human cerebral cortex (Fig. 1). Therefore, all encephalopathic features of the disease emerging by mutations in this gene originate solely from the neuronal cell population.
- Lambeth DO, Tews KN, Adkins S, Frohlich D, Milavetz BI (2004) Expression of two succinyl-CoA synthetases with different nucleotide specificities in mammalian tissues. J Biol Chem 279: 36621-36624.
- Ostergaard E (2008) Disorders caused by deficiency of succinate-CoA ligase. J Inherit Metab Dis 31: 226-229.
- Carrozzo R, Onisi-Vici C, Steuerwald U, Lucioli S, Deodato F, Di GS, Bertini E, Franke B, Kluijtmans LA, Meschini MC, Rizzo C, Piemonte F, Rodenburg R, Santer R, Santorelli FM, van RA, Vermunt-de KD, Morava E, Wevers RA (2007) SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain 130: 862-874.
• Keywords: SUCLA2, Mitochondrial disorder, Cortex
• O2k-Network Lab: HU Budapest Chinopoulos C
Labels:
Stress:Mitochondrial disease Organism: Human Tissue;cell: Nervous system
Affiliations and author contributions
Arpád Dobolyi (1), Elsebet Ostergaard (2), Attila G. Bagó (1,3), Miklós Palkovits (1,4), Vera Adam-Vizi (5) and Christos Chinopoulos (5)
(1) Department of Anatomy, Histology and Embryology, Semmelweis University, Hungary; Email: [email protected]
(2) Department of Clinical Genetics, Copenhagen University Hospital Rigshospitalet, Denmark
(3) National Institute of Neurosurgery, Hungary
(4) Human Brain Tissue Bank, Semmelweis University, Hungary
(5) Department of Medical Biochemistry, Semmelweis University, Hungary
Figure 1
Co-localization of fluorescent Nissl staining the cytosol of neurons (red)
and the ADP-forming β-subunit (A-SUCL-β)
of succinyl CoA ligase (green-->yellow) in human temporal cortex.