Chen 2022 Am J Physiol Cell Physiol
| Chen CL, Zhang L, Jin Z, Kasumov T, Chen YR (2022) Mitochondrial redox regulation and myocardial ischemia-reperfusion injury. Am J Physiol Cell Physiol 322:C12-23. doi: 10.1152/ajpcell.00131.2021 |
Chen CL, Zhang L, Jin Z, Kasumov T, Chen YR (2022) Am J Physiol Cell Physiol
Abstract: Mitochondrial reactive oxygen species (ROS) have emerged as an important mechanism of disease and redox signaling in the cellular system. Under basal or pathological conditions, electron leakage for ROS production is primarily mediated by complexes I and III of the electron transport chain (ETC) and by the proton motive force (PMF), consisting of a membrane potential (ΔΨ) and a proton gradient (ΔpH). Several factors control redox status in mitochondria, including ROS, the PMF, oxidative posttranslational modifications (OPTM) of the ETC subunits, SOD2, and cytochrome c heme lyase (HCCS). In the mitochondrial PMF, increased ΔpH-supported backpressure due to diminishing electron transport and chemiosmosis promotes a more reductive mitochondrial physiological setting. OPTM by protein cysteine sulfonation in complex I and complex III has been shown to affect enzymatic catalysis, the proton gradient, redox status, and enzyme-mediated ROS production. Pathological conditions associated with oxidative or nitrosative stress, such as myocardial ischemia and reperfusion (I/R), increase mitochondrial ROS production and redox dysfunction via oxidative injury to complexes I and III, intensely enhancing protein cysteine sulfonation and impairing heme integrity. The physiological conditions of reductive stress induced by gains in SOD2 function normalize I/R-mediated ROS overproduction and redox dysfunction. Further insight into the cellular mechanisms by which HCCS, biogenesis of c-type cytochrome, and OPTM regulate PMF and ROS production in mitochondria will enrich our understanding of redox signal transduction and identify new therapeutic targets for cardiovascular diseases in which oxidative stress perturbs normal redox signaling.
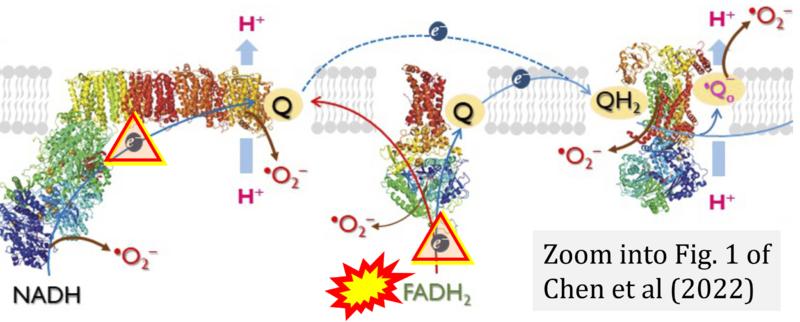
Correction: FADH2 and S-pathway
- A commonly found error on FADH2 in the S-pathway requires correction. For clarification, see page 48 in Gnaiger (2020)
- Quote (p 48): "The substrate of CII is succinate, which is oxidized forming fumarate while reducing flavin adenine dinucleotide FAD to FADH2, with further electron transfer to the quinone pool. Whereas reduced NADH is a substrate of Complex I linked to dehydrogenases of the TCA cycle and mt-matrix upstream of CI, reduced FADH2 is a product of Complex II with downstream electron flow from CII to Q."
- A commonly found error on FADH2 in the S-pathway requires correction. For clarification, see page 48 in Gnaiger (2020)
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
Labels:
Stress:Ischemia-reperfusion, Oxidative stress;RONS
Tissue;cell: Heart
Regulation: Redox state