Avram 2021 Int J Mol Sci
| Avram VF, Chamkha I, Åsander-Frostner E, Ehinger JK, Timar RZ, Hansson MJ, Muntean DM, Elmér E (2021) Cell-permeable succinate rescues mitochondrial respiration in cellular models of statin toxicity. Int J Mol Sci 22:424. |
» PMID: 33401621 Open Access »![]()
Avram Vlad F, Chamkha Imen, Aasander-Frostner Eleonor, Ehinger Johannes K, Timar Romulus Z, Hansson Magnus J, Muntean Danina M, Elmer Eskil (2021) Int J Mol Sci
Abstract: Statins are the cornerstone of lipid-lowering therapy. Although generally well tolerated, statin-associated muscle symptoms (SAMS) represent the main reason for treatment discontinuation. Mitochondrial dysfunction of complex I has been implicated in the pathophysiology of SAMS. The present study proposed to assess the concentration-dependent ex vivo effects of three statins on mitochondrial respiration in viable human platelets and to investigate whether a cell-permeable prodrug of succinate (complex II substrate) can compensate for statin-induced mitochondrial dysfunction. Mitochondrial respiration was assessed by high-resolution respirometry in human platelets, acutely exposed to statins in the presence/absence of the prodrug NV118. Statins concentration-dependently inhibited mitochondrial respiration in both intact and permeabilized cells. Further, statins caused an increase in non-ATP generating oxygen consumption (uncoupling), severely limiting the OXPHOS coupling efficiency, a measure of the ATP generating capacity. Cerivastatin (commercially withdrawn due to muscle toxicity) displayed a similar inhibitory capacity compared with the widely prescribed and tolerable atorvastatin, but did not elicit direct complex I inhibition. NV118 increased succinate-supported mitochondrial oxygen consumption in atorvastatin/cerivastatin-exposed platelets leading to normalization of coupled (ATP generating) respiration. The results acquired in isolated human platelets were validated in a limited set of experiments using atorvastatin in HepG2 cells, reinforcing the generalizability of the findings. • Keywords: HepG2 cells, NV118, Cell-permeable succinate, Mitochondria, MitoKit-CII, Platelets, Statins • Bioblast editor: Plangger M • O2k-Network Lab: RO Timisoara Muntean DM, SE Lund Elmer E
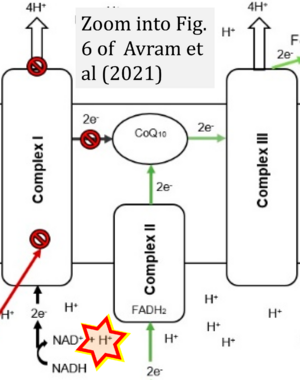
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels: MiParea: Respiration, Pharmacology;toxicology
Organism: Human
Tissue;cell: Platelet
Preparation: Intact cells
Coupling state: LEAK, ROUTINE, ET
Pathway: ROX
HRR: Oxygraph-2k
2021-01, MitoKit-CII, RO, SE, O2k-brief