Difference between revisions of "Advancement"
| Line 13: | Line 13: | ||
|info=[[Gnaiger_1993_Pure Appl Chem |Gnaiger (1993) Pure Appl Chem]], [[Gnaiger 2020 BEC MitoPathways]] | |info=[[Gnaiger_1993_Pure Appl Chem |Gnaiger (1993) Pure Appl Chem]], [[Gnaiger 2020 BEC MitoPathways]] | ||
}} | }} | ||
Communicated by [[Gnaiger E]] ( | Communicated by [[Gnaiger E]] (2018-11-02) last update 2021-01-27 | ||
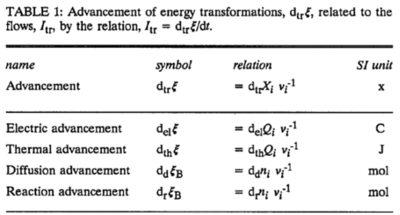
[[File:Advancement Table 1 Gnaiger 1993 Pure Appl Chem.png|400px|link=Gnaiger 1993 Pure Appl Chem |thumb|d<sub>el</sub>''Q''<sub>''i''</sub> (d<sub>th</sub>''Q''<sub>''i''</sub>) are the changes in electric charge (heat) at the compartments of high or low electric potential (temperature) within the discontinuous system (from ref. [2]).]] | |||
== Synonyms == | |||
::::* advancement | |||
::::* extent (of reaction) | |||
::::* displacement (of a moving particle) | |||
== Advancement per volume == | == Advancement per volume == | ||
:::: The advancement of a transformation in a closed homogenous system (chemical reaction) or discontinuous system (diffusion) causes a change of [[concentration]] of substances ''i''. | :::: The advancement of a transformation in a closed homogenous system (chemical reaction) or discontinuous system (diffusion) causes a change of [[concentration]] of substances ''i''. | ||
:::: The advancement causes a ''change'' of concentration due to a transformation, Δ<sub>tr</sub>''c'', in contrast to a difference of concentrations calculated between difference states, Δ<sub>tr</sub>''c''. | :::: The advancement causes a ''change'' of concentration due to a transformation, Δ<sub>tr</sub>''c'', in contrast to a difference of concentrations calculated between difference states, Δ<sub>tr</sub>''c''. | ||
::::» [[Advancement per volume]], d<sub>tr</sub>''Y'' = d<sub>tr</sub>''ξ'' | ::::» [[Advancement per volume]], d<sub>tr</sub>''Y'' = d<sub>tr</sub>''ξ''∙''V''<sup>-1</sup> | ||
| Line 27: | Line 32: | ||
:::# Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65:1983-2002. - [[Gnaiger 1993 Pure Appl Chem |»Bioblast link«]] | :::# Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65:1983-2002. - [[Gnaiger 1993 Pure Appl Chem |»Bioblast link«]] | ||
:::# Prigogine I (1967) Introduction to thermodynamics of irreversible processes. Interscience New York, 3rd ed:147 pp. - [[Prigogine 1967 Interscience |»Bioblast link«]] | :::# Prigogine I (1967) Introduction to thermodynamics of irreversible processes. Interscience New York, 3rd ed:147 pp. - [[Prigogine 1967 Interscience |»Bioblast link«]] | ||
:::# Grosholz Emily R (2007) Representation and productive ambiguity in mathematics and the sciences. Oxford Univ Press 312 pp. - [[Prigogine 1967 Interscience |»Bioblast link«]] — ".. distance that is re-conceptualized to mean displacement, since we are instructed to suppose that a moving particle is traversing it." | |||
{{Keywords: Force and membrane potential}} | {{Keywords: Force and membrane potential}} | ||
Revision as of 14:37, 27 January 2021
Description
In an isomorphic analysis, any form of flow is the advancement of a process per unit of time, expressed in a specific motive unit [MU∙s-1], e.g., ampere for electric flow or current, Iel = delξ/dt [A≡C∙s-1], watt for thermal or heat flow, Ith = dthξ/dt [W≡J∙s-1], and for chemical flow of reaction, Ir = drξ/dt, the unit is [mol∙s-1] (extent of reaction per time). The corresponding motive forces are the partial exergy (Gibbs energy) changes per advancement [J∙MU-1], expressed in volt for electric force, ΔelF = ∂G/∂elξ [V≡J∙C-1], dimensionless for thermal force, ΔthF = ∂G/∂thξ [J∙J-1], and for chemical force, ΔrF = ∂G/∂rξ, the unit is [J∙mol-1], which deserves a specific acronym [Jol] comparable to volt [V]. For chemical processes of reaction (spontaneous from high-potential substrates to low-potential products) and compartmental diffusion (spontaneous from a high-potential compartment to a low-potential compartment), the advancement is the amount of motive substance that has undergone a compartmental transformation [mol]. The concept was originally introduced by De Donder [1]. Central to the concept of advancement is the stoichiometric number, νi, associated with each motive component i (transformant [2]).
In a chemical reaction r the motive entity is the stoichiometric amount of reactant, drni, with stoichiometric number νi. The advancement of the chemical reaction, drξ [mol], is defined as,
drξ = drni·νi-1
The flow of the chemical reaction, Ir [mol·s-1], is advancement per time,
Ir = drξ·dt-1
This concept of advancement is extended to compartmental diffusion and the advancement of charged particles [3], and to any discontinuous transformation in compartmental systems [2],
Abbreviation: dtrξ [MU]
Reference: Gnaiger (1993) Pure Appl Chem, Gnaiger 2020 BEC MitoPathways
Communicated by Gnaiger E (2018-11-02) last update 2021-01-27
Synonyms
- advancement
- extent (of reaction)
- displacement (of a moving particle)
Advancement per volume
- The advancement of a transformation in a closed homogenous system (chemical reaction) or discontinuous system (diffusion) causes a change of concentration of substances i.
- The advancement causes a change of concentration due to a transformation, Δtrc, in contrast to a difference of concentrations calculated between difference states, Δtrc.
- » Advancement per volume, dtrY = dtrξ∙V-1
References
- De Donder T, Van Rysselberghe P (1936) Thermodynamic theory of affinity: a book of principles. Oxford, England: Oxford University Press:144 pp.
- Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65:1983-2002. - »Bioblast link«
- Prigogine I (1967) Introduction to thermodynamics of irreversible processes. Interscience New York, 3rd ed:147 pp. - »Bioblast link«
- Grosholz Emily R (2007) Representation and productive ambiguity in mathematics and the sciences. Oxford Univ Press 312 pp. - »Bioblast link« — ".. distance that is re-conceptualized to mean displacement, since we are instructed to suppose that a moving particle is traversing it."
- Bioblast links: Force and membrane potential - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Fundamental relationships
- mt-Membrane potential and protonmotive force
- O2k-Potentiometry
- » O2k-Catalogue: O2k-TPP+ ISE-Module
- » O2k-Manual: MiPNet15.03 O2k-MultiSensor-ISE
- » TPP - O2k-Procedures: Tetraphenylphosphonium
- » Specifications: MiPNet15.08 TPP electrode
- » Poster
- » Unspecific binding of TPP+
- » TPP+ inhibitory effect
- O2k-Potentiometry
- O2k-Fluorometry
- » O2k-Catalogue: O2k-FluoRespirometer
- » O2k-Manual: MiPNet22.11 O2k-FluoRespirometer manual
- » Safranin - O2k-Procedures: MiPNet20.13 Safranin mt-membranepotential / Safranin
- » TMRM - O2k-Procedures: TMRM
- O2k-Fluorometry
- O2k-Publications
- Bioblast links: Normalization - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Quantities for normalization
- » Count in contrast to Number
- » Mitochondrial marker
- » O2k-Protocols: mitochondrial and marker-enzymes
- » Citrate synthase activity
- Quantities for normalization
- General
- Related keyword lists
MitoPedia concepts:
MiP concept,
Ergodynamics