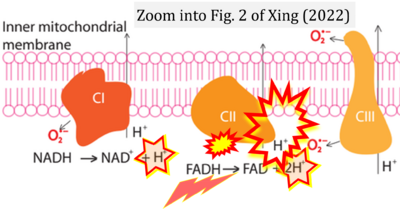
Xing 2022 Atlantis Press
| Xing Yunxie (2022) Is genome instability a significant cause of aging? A review. Advances in Social Science, Education and Humanities Research 664, Atlantis Press. https://doi.org/10.2991/assehr.k.220504.260 |
Xing Yunxie (2022) Advances in Social Science, Education and Humanities Research, Atlantis Press
Abstract: What is the main cause of aging has been discussed over last decades. Accumulating evidences has indicated that genome instability including mitochondrial DNA in somatic cell played an important role in human aging process. Various sources of damage such as reactive oxygen species and UV radiation can lead to the decrease of DNA robustness. In addition, the erroneous replication and failure in DNA maintenance system is also able to casus mutations and epimutations. In this review we will first summarize the relation between the accumulation of DNA damage and longevity. Then we will review the contribution made by mitochondrial free radical to the aging process. Finally, we will briefly discuss whether the telomere is an eligible biomarker of life span.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase