Weidgang 2015 Abstract MiP2015
| Mitochondrial respiration in homogenized biopsies from kidney, liver, heart and diaphragm from mice after blunt thoracic trauma and hemorrhagic shock. Effects of a novel hydrogen sulfide donor AP39. |
Link:
Weidgang C, Groeger M, Weber S, Radermacher P, Calzia E (2015)
Event: MiP2015
Haemorrhagic shock impairs the perfusion and subsequently the oxygenation of crucial organs as for heart, kidney and liver. The combination of haemorrhagic shock after thoracic trauma increases this effect. Here we tested whether the mitochondrial respiration is impaired by these conditions and whether the treatment with a novel synthesised mitochondrially-targeted hydrogen sulfide (H2S) donor, AP39, has beneficial effects on mitochondrial respiration. In fact, AP39 has previously been shown to increase mitochondrial activity, however this data has only been obtained in vitro so far [1].
This project illustrates preliminary data in terms of mitochondrial respiration in organs suffering from haemorrhagic shock combined with thoracic trauma and will further reveal the impact of AP39 in this setting.
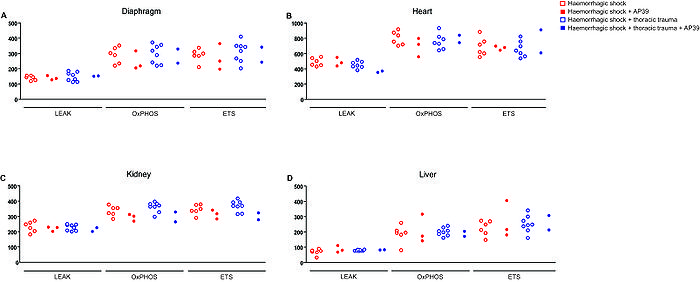
Mitochondrial function was measured in small homogenised samples from diaphragm, heart, kidney and liver from 3-6 mice and measured in terms of LEAK-, OXPHOS- and ET-capacity by using an O2k-Oxygraph (Oroboros Instruments, Austria). Respiratory activity of the samples was simultaneously supported by complex I and II substrates (Malate, Glutamate, Pyruvat and Succinate), as well as by ADP and quantified in terms of oxygen flux [JO2] per wet weight with the unit of pmol O2/ (mg*s). OXPHOS-capacity was obtained as the maximum activity under all substrates and ADP, LEAK-capacity by inhibition of the ATP-synthase obtained by further injection of oligomycine and finally, ET-capacity by addition of the mitochondrial chain uncoupler FCCP.
Here we present preliminary data with almost descriptive statistical analyses. We can determine minor changes in mitochondrial function upon haemorrhagic shock and in combination with thoracic trauma. However, upon the addition of AP39 the mitochondrial activity seems to decrease, especially in the tissue of liver and heart.
Our test provides reliable data on mitochondrial respiration in various tissues thus allowing an overview of global effects in the whole organism. In fact, our data suggest that organs respond differently to severe haemorrhagic shock and the combination with thoracic trauma, as well to the treatment with AP39. In contrast to previous data, we did not observe an increase of mitochondrial activity under AP39 instead mitochondrial activation tends to decrease after treatment with the sulfide donor. The physiological meaning of this effect required further investigation.
• O2k-Network Lab: DE Ulm Radermacher P
Labels: MiParea: Respiration, Comparative MiP;environmental MiP
Stress:Ischemia-reperfusion Organism: Mouse Tissue;cell: Heart, Skeletal muscle, Liver, Kidney Preparation: Homogenate
Coupling state: LEAK, OXPHOS, ET
Pathway: N, S
HRR: Oxygraph-2k
Event: A1, Oral
MiP2015
Affiliations
Inst Anaesthesiological Pathophysiology Process Development, Ulm Univ Hospital, Germany. - [email protected]
Figure 1
Figure 1. Distinct organ responses following severe haemorrhagic shock or in combination with thoracic trauma. The mitochondrial respiration of crucially affected organs during haemorrhagic shock (diaphragm (A), heart (B), kidney (C) and liver (D)) is variously affected by either single haemorrhagic shock or in combination with thoracic trauma. Notably, the incubation with a novel synthesised mitochondrially-targeted hydrogen sulfide (H2S) donor, AP39, leads to a decrease of mitochondrial respiration suggesting beneficial effects. Abbreviations: LEAK: Respiration in the absence of ADP due to uncoupled respiration but under maintained oxygen flux; OXPHOS capacity: maximal oxidative phosphorylation activity; ET capacity: mitochondrial electron transfer-pathway.
References
- Szczesny B, Modis K, Yanagi K, Coletta C, Le Trionnaire S, Perry A, Wood M E, Whiteman M, Szabo C (2014) AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 41:120-30.