Torres 2018 Cell Metab
| Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, Lowe DA, Spangenburg EE, Zeczycki TN, Shaikh SR, Neufer PD (2018) 17β-estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab 27:167-79. https://doi.org/10.1016/j.cmet.2017.10.003 |
Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, Lowe DA, Spangenburg EE, Zeczycki TN, Shaikh SR, Neufer PD (2018) Cell Metab
Abstract: Menopause results in a progressive decline in 17β-estradiol (E2) levels, increased adiposity, decreased insulin sensitivity, and a higher risk for type 2 diabetes. Estrogen therapies can help reverse these effects, but the mechanism(s) by which E2 modulates susceptibility to metabolic disease is not well understood. In young C57BL/6N mice, short-term ovariectomy decreased-whereas E2 therapy restored-mitochondrial respiratory function, cellular redox state (GSH/GSSG), and insulin sensitivity in skeletal muscle. E2 was detected by liquid chromatography-mass spectrometry in mitochondrial membranes and varied according to whole-body E2 status independently of ERα. Loss of E2 increased mitochondrial membrane microviscosity and H2O2 emitting potential, whereas E2 administration in vivo and in vitro restored membrane E2 content, microviscosity, complex I and I + III activities, H2O2 emitting potential, and submaximal OXPHOS responsiveness. These findings demonstrate that E2 directly modulates membrane biophysical properties and bioenergetic function in mitochondria, offering a direct mechanism by which E2 status broadly influences energy homeostasis. • Keywords: Estrogen, Hormone replacement therapy, Hydrogen peroxide, Insulin resistance, Membrane viscosity, Menopause, Mitochondria, Ovariectomy, Buffer Z, Blebbistatin, Amplex UltraRed • Bioblast editor: Kandolf G • O2k-Network Lab: US NC Greenville Neufer PD
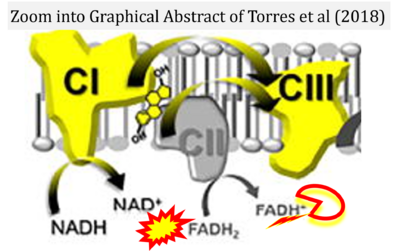
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: MiParea: Respiration, mt-Medicine, Pharmacology;toxicology Pathology: Diabetes
Organism: Mouse Tissue;cell: Skeletal muscle Preparation: Permeabilized tissue
Coupling state: LEAK, OXPHOS, ET
Pathway: F, N, S, CIV, NS, ROX
HRR: Oxygraph-2k
2018-01