Schoepf 2014 Abstract MiP2014
| OXPHOS analysis in small prostate biopsies. |
Link:
Mitochondr Physiol Network 19.13 - MiP2014
Schoepf Bernd, Schaefer G, Gnaiger E, Klocker H (2014)
Event: MiP2014
In 2012, adenocarcinoma of the prostate was the most prevalent male cancer type in Western civilized countries, accounting for 9.5% of all cancer related deaths in Europe [1,2].
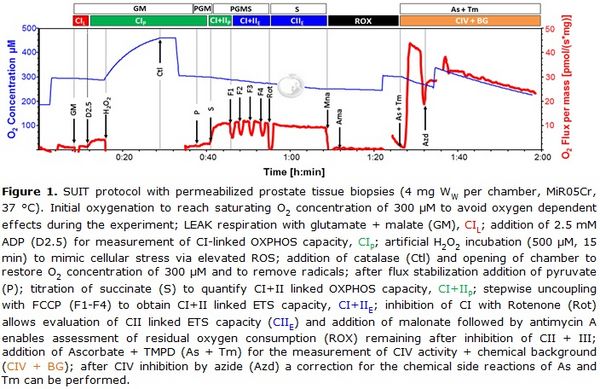
Numerous studies have addressed the metabolic characteristics and molecular pathways of the disease, primarily applying prostate cancer related cell lines as models. In contrast, very little is known about the metabolic properties of fresh prostate cancer tissue in terms of mitochondrial oxidative phosphorylation (OXPHOS) and ATP production. This lack of data is mainly due to the limited access to fresh prostate tissue biopsies and their small sample size. Therefore, in order to overcome this limitation, we developed a method which, for the first time, enables OXPHOS analysis of very small benign and malignant prostate tissue biopsies (2-5 mg wet weight per individual measurement). Samples were harvested immediately after radical prostatectomy and examined using high-resolution-respirometry (Oroboros Oxygraph-2k) and a sophisticated substrate-uncoupler-inhibitor titration (SUIT) protocol. For this purpose, prostate tissue was mechanically permeabilized which, as previously reported, represents a very good mitochondrial preparation alternative to isolated mitochondria, both reducing the amount of required sample material and largely preserving structural integrity of the cell [3]. An artificial incubation with H2O2 was used to assess the mitochondrial response to cellular stress exerted by enhanced ROS production [4]. In addition, an easy and fast assay for determination of CIV activity was applied adding ascorbate and the CIV substrate N,N,N',N'-Tetramethyl-p-phenylenediamine dihydrochloride (TMPD) followed by inhibition with sodium azide to correct for chemical background reactions. The SUIT protocol, including different substance combinations, allows measurement of flux control variables in different substrate and coupling states (Fig. 1).
Any sample biopsy (app. 6–10 mg of wet weight) was divided into two subsamples and mechanically permeabilized using two pairs of extra-sharp forceps [5]. Each subsample was placed into one of the four chambers of two Oroboros Oxygraph-2k operated in parallel. Measurements were performed in MiR05 with creatine (MiR05Cr) at 37 °C. Parallel analyses of a paired sample consisting of one benign and one malign biopsy from a single patient could be conducted within little more than two hours, yielding high quality respirometry data while preserving tissue structure for subsequent tissue analysis and DNA extraction.
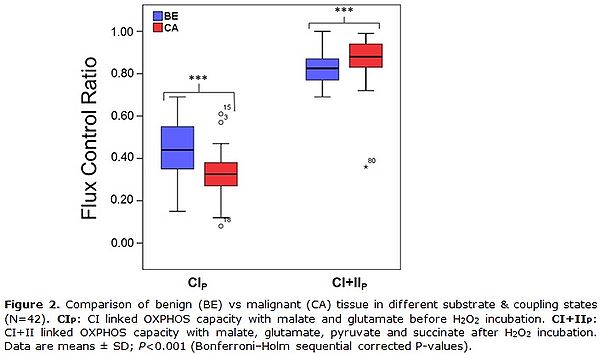
The first data evaluations revealed an overall decreased CI-linked OXPHOS capacity before H2O2 treatment, a higher vulnerability toward cellular stress mediated by H2O2 but a similar CI&II-linked OXPHOS capacity after H2O2 treatment in cancer compared to benign tissue (Fig. 2).
• O2k-Network Lab: AT Innsbruck Oroboros
Affiliation
1-Oroboros Instruments; 2-Oncotyrol – Center Personalized Cancer Medicine; 3-Div Genetic Epidemiology*; 4-Dep Urology*; 5-Dep Visceral, Transplant Thoracic Surgery, Daniel Swarovski Research Lab*, *Medical Univ Innsbruck; Innsbruck, Austria. - [email protected]
Figures
Figure 1. SUIT protocol with permeabilized prostate tissue biopsies (4 mg Ww per chamber, MiR05Cr, 37 °C). Initial oxygenation to reach O2 concentration of 300 µM to avoid oxygen dependent effects during the experiment; LEAK respiration with glutamate + malate (GM), CIL; addition of 2.5 mM ADP (D2.5) for measurement of CI-linked OXPHOS capacity, CIP; H2O2 incubation (500 µM, 15 min) to induce oxidative stress; termination of oxidative stress by addition of catalase (Ctl) and opening of chamber to restore O2 concentration of 300 µM; after flux stabilization addition of pyruvate (P); titration of succinate (S) to quantify CI+&II-linked OXPHOS capacity, CI&IIP; stepwise uncoupling with FCCP (F1-F4) to obtain CI&II-linked ET capacity, CI&IIE; inhibition of CI with Rotenone (Rot) allows evaluation of CII-linked ET capacity (CIIE) and addition of malonate followed by antimycin A enables assessment of residual oxygen consumption (ROX) remaining after inhibition of CII and CIII; addition of Ascorbate + TMPD (As + Tm) for the measurement of CIV activity and chemical background (CIV + BG); after CIV inhibition by azide (Azd) a correction for the chemical side reactions of As and Tm can be performed.
Figure 2. Comparison of benign (BE) vs malignant (CA) tissue in different substrate & coupling states (N=42). CIP: CI-linked OXPHOS capacity with malate and glutamate before H2O2 incubation. CI&I P: CI&II-linked OXPHOS capacity with malate, glutamate, pyruvate and succinate after H2O2 incubation. Data are means ± SD; P<0.001 (Bonferroni–Holm sequential corrected P-values).
References and acknowledgements
Supported by Oncotyrol.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374-403.
- Siegel R, Naishadham D, Jemal A (2012) Cancer statistics (2012) CA Cancer J Clin 62: 10-29.
- Pesta D, Gnaiger E (2012) High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25-58.
- Stadlmann S, Rieger G, Amberger A, Kuznetsov AV, Margreiter R, Gnaiger E (2002) H2O2-mediated oxidative stress versus cold ischemia-reperfusion: mitochondrial respiratory defects in cultured human endothelial cells. Transplantation 74: 1800-3.
- Kuznetsov AV, Strobl D, Ruttmann E, Konigsrainer A, Margreiter R, Gnaiger E (2002) Evaluation of mitochondrial respiratory function in small biopsies of liver. Anal Biochem 305: 186-94.
Labels: MiParea: Respiration, Patients
Pathology: Cancer
Stress:Oxidative stress;RONS
Organism: Human
Tissue;cell: Genital
Preparation: Permeabilized tissue
Coupling state: LEAK, OXPHOS, ET
Pathway: N, S, CIV, NS
HRR: Oxygraph-2k
Event: A4, Oral
MiP2014