Schinagl 2016 PLoS One
| Schinagl CW, Vrabl P, Burgstaller W (2016) Adapting high-resolution respirometry to glucose-limited steady state mycelium of the filamentous fungus Penicillium ochrochloron: method development and standardisation. PLoS One 11:e0146878. |
Schinagl C, Vrabl P, Burgstaller W (2016) PLoS One
Abstract: Fungal electron transport systems (ET-pathway) are branched, involving alternative NADH dehydrogenases and an alternative terminal oxidase. These alternative respiratory enzymes were reported to play a role in pathogenesis, production of antibiotics and excretion of organic acids. The activity of these alternative respiratory enzymes strongly depends on environmental conditions. Functional analysis of fungal ET-pathway under highly standardised conditions for cultivation, sample processing and respirometric assay are still lacking. We developed a highly standardised protocol to explore in vivo the ET-pathway -and in particular the alternative oxidase-in Penicillium ochrochloron. This included cultivation in glucose-limited chemostat (to achieve a defined and reproducible physiological state), direct transfer without any manipulation of a broth sample to the respirometer (to maintain the physiological state in the respirometer as close as possible to that in the chemostat), and high-resolution respirometry (small sample volume and high measuring accuracy). This protocol was aimed at avoiding any changes in the physiological phenotype due to the high phenotypic plasticity of filamentous fungi. A stable oxygen consumption (< 5% change in 20 minutes) was only possible with glucose limited chemostat mycelium and a direct transfer of a broth sample into the respirometer. Steady state respiration was 29% below its maximum respiratory capacity. Additionally to a rotenone-sensitive complex I and most probably a functioning complex III, the ET-pathway of P. ochrochloron also contained a cyanide-sensitive terminal oxidase (complex IV). Activity of alternative oxidase was present constitutively. The degree of inhibition strongly depended on the sequence of inhibitor addition. This suggested, as postulated for plants, that the alternative terminal oxidase was in dynamic equilibrium with complex IV-independent of the rate of electron flux. This means that the onset of activity does not depend on a complete saturation or inhibition of the cytochrome pathway. • Keywords: Penicillium ochrochloron, Alternative oxidase
• O2k-Network Lab: AT Innsbruck Burgstaller W
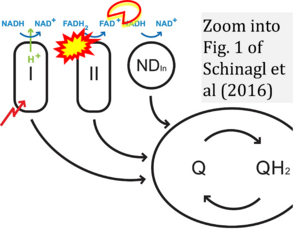
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: MiParea: Respiration
Organism: Fungi
Preparation: Intact cells Enzyme: Complex I, Complex III, Complex IV;cytochrome c oxidase
Coupling state: ROUTINE, ET
HRR: Oxygraph-2k
2016-01