Description
Reference: A: protocol for simultaneous determination of O2 flux and NADH autofluorescence in mitochondrial preparations (isolated mitochondria, tissue homogenate and permeabilized cells)- SUIT-006
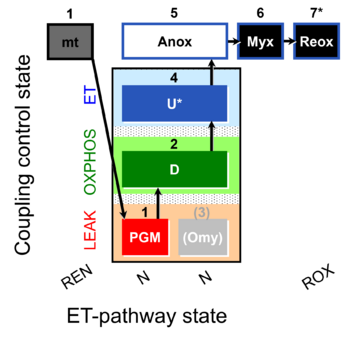
SUIT number: D084_mt;1PGM;2D;3(Omy);4U;5Anox;6Myx;7Reox
O2k-Application: NADH
The coupling-control protocol SUIT-006 NADH mt D084 allows the study of mitochondrial respiration and NADH fluorescence in the three coupling control states LEAK, OXPHOS and ET in the N-pathway.
After the addition of mitochondria in the absence of fuel substrates and ADP, Ren, respiration due to oxidation of endogenous substrates remaining after mitochondrial isolation is measured. If these substrates are fully consumed by the mitochondria, this step can be used for an approximate calibration of oxidized NAD (NAD defined as the sum of the oxidized NAD+ and the reduced NADH). If this is not possible, this protocol should be used in combination with SUIT-034 NADH mt D082, where the titration of a small concentration of ADP leads to depletion of endogenous substrates, thus leading to accumulation of oxidized NAD, allowing to calibrate for the fully oxidized NAD.
Anoxia is reached by letting mitochondria fully consume the oxygen in the O2k-chambers. In the absence of O2, the ETS upstream of CIV is reduced and thus leads to an accumulation of reduced NAD. Under anoxia the complex III inhibitor myxothiazol is added and a further increase in the reduced NAD fraction can be observed. This step is then used for the calibration of the fully reduced NAD. At the end of the protocol, the reoxigenation of the chamber allows the measurement of Rox.
Communicated by Grings M, Cardoso Luiza HD (last update 2023-12-21)
Representative traces
Steps and respiratory states
| Step | State | Pathway | Q-junction | Comment - Events (E) and Marks (M) |
|---|---|---|---|---|
| mt | REN | mt | ||
| 1PGM | PGML(n) | N | CI | 1PGM
|
| 2D | PGMP | N | CI | 1PGM;2D
|
| (3Omy) | PGML(Omy) | N | CI | 1PGM;2D;(3Omy)
|
| 4U | PGME | N | CI | 1PGM;2D;(3Omy);4U
|
| 5Anox | N | CI | 1PGM;2D;(3Omy);4U;5Anox | |
| 6Myx | N | CI | 1PGM;2D;(3Omy);4U;5Anox;6Myx
| |
| 7Reox | ROX | 1PGM;2D;(3Omy);4U;5Anox;6Myx;7Reox |
- Bioblast links: SUIT protocols - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Coupling control
- Pathway control
- Main fuel substrates
- » Glutamate, G
- » Glycerophosphate, Gp
- » Malate, M
- » Octanoylcarnitine, Oct
- » Pyruvate, P
- » Succinate, S
- Main fuel substrates
- Glossary
Strengths and limitations
- SUIT-006 NADH mt D084 in combination with SUIT-034 NADH mt D082 provides NAD redox ratios in LEAK, OXPHOS and ET states, measured simultaneously with respiration.
- + Reasonable duration of the experiment.
- + H2 gas from Oxia or N2/argon can be used to decrease O2 concentration to obtain anoxia faster.
- - Fully oxidized NAD can only be obtained with the combination with SUIT-034 NADH mt D082 or with samples in which endogenous substrates are absent.
- - Careful washing is required after the experiment to avoid carry-over of uncoupler and inhibitors. The addition of liver homogenate is recommended in the washing protocol to bind strong inhibitors.
- - The concentration of the oxidized and reduced NAD fraction cannot be determined.
- - Omy concentration has to be determined if used. Higher concentrations of Omy may inhibit the ET state.
- - Antimycin A and CCCP cannot be used due to the high chemical background effect on fluorescence.
- - Cytochrome c test cannot be performed during the protocol as it affects fluorescence. Cytochrome c test can be performed in the following protocol: SUIT-006 O2 mt D108.
- After myxothyazol titration, this protocol can be extended with the Complex IV assay.
Compare SUIT protocols
- SUIT-032 NADH mt D078: Protocol for simultaneous determination of O2 flux and NADH autofluorescence in isolated mitochondria. Similar protocol without uncoupler titrations and ET state evaluation.
- SUIT-034 NADH mt D082: Protocol for simultaneous determination of O2 flux and NADH autofluorescence in isolated mitochondria, allowing for calibration of the NAD redox ratios with samples that contain residual endogenous substrates. Additional titration of low concentration of ADP (0.1 μM) for depletion of endogenous substrates and calibration of fully reduced NAD, allowing for cross-calibration with SUIT-006 NADH mt D084.
- SUIT-006 O2 mt D108: Control protocol for respiration only, allowing for cytochrome c test.
Chemicals and syringes
| Step | Chemical(s) and link(s) | Comments |
|---|---|---|
| 1PGM | Pyruvate (P), Glutamate (G), and Malate (M) | |
| 2D | ADP (D) | |
| (3Omy) | Oligomycin (Omy) | This step can be skipped. |
| 4U | SF6847 | We do not recommend the use of any other uncoupler, like Carbonyl cyanide m-chlorophenyl hydrazone, CCCP (U), due to the chemical background effect on fluorescence. |
| 5Anox | The O2 concentration in the O2k-chamber can be decreased by N2 or H2 injection to reach faster anoxia, see: Setting the oxygen concentration. | |
| 6Myx | Myxothiazol | We do not recommend the use of any other inhibitor of complex III, like Antimycin A (Ama), due to the chemical background effect on fluorescence. |
| 7Reox | Reoxygenation can be performed by opening the chamber, see: Open chamber. |
- Suggested stock concentrations are shown in the specific DL-Protocol.