Nsiah-Sefaa 2016 Biosci Rep
| Nsiah-Sefaa A, McKenzie M (2016) Combined defects in oxidative phosphorylation and fatty acid β-oxidation in mitochondrial disease. Biosci Rep 36:e00313. https://doi.org/10.1042/BSR20150295 |
Nsiah-Sefaa A, McKenzie M (2016) Biosci Rep
Abstract: Mitochondria provide the main source of energy to eukaryotic cells, oxidizing fats and sugars to generate ATP. Mitochondrial fatty acid β-oxidation (FAO) and oxidative phosphorylation (OXPHOS) are two metabolic pathways which are central to this process. Defects in these pathways can result in diseases of the brain, skeletal muscle, heart and liver, affecting approximately 1 in 5000 live births. There are no effective therapies for these disorders, with quality of life severely reduced for most patients. The pathology underlying many aspects of these diseases is not well understood; for example, it is not clear why some patients with primary FAO deficiencies exhibit secondary OXPHOS defects. However, recent findings suggest that physical interactions exist between FAO and OXPHOS proteins, and that these interactions are critical for both FAO and OXPHOS function. Here, we review our current understanding of the interactions between FAO and OXPHOS proteins and how defects in these two metabolic pathways contribute to mitochondrial disease pathogenesis.
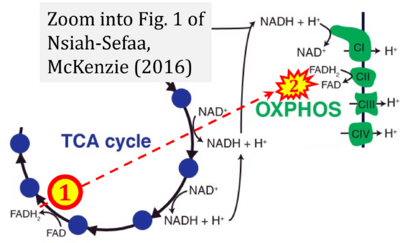
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: MiParea: mt-Medicine
Enzyme: Complex II;succinate dehydrogenase
Pathway: F