Laner 2014 Abstract MiP2014
| Cytochrome c flux control factor as a quality criterion in respiratory OXPHOS analysis in canine permeabilized fibers. |
Link:
Mitochondr Physiol Network 19.13 - MiP2014
Laner V, Boushel RC, Hamilton KL, Miller BF, Williamson KK, Davis MS, Gnaiger E (2014)
Event: MiP2014
Mitochondrial (mt) preparations (isolated mitochondria, permeabilized cells and tissues, tissue homogenates) provide a fundamental basis for comprehensive OXPHOS analysis for the study of substrate and coupling control of mitochondrial respiration [1]. Plasma membrane permeabilization with mechanical separation of muscle fiber bundles and chemical permeabilization with mild detergents may influence the integrity of the outer mt-membrane and thus induce partial release of cytochrome c (c). In mitochondria isolated from healthy skeletal muscle, CI&II-linked OXPHOS capacity decreases linearly with cytochrome c loss during isolation [2]. The cytochrome c effect is expressed as the flux control factor FCFc, which is the increase of OXPHOS capacity after addition of 10 µM c normalized for c-stimulated respiration [1-3]. There is no consensus as to the threshold of FCFc applied as a quantitative exclusion criterion in permeabilized fibers obtained from healthy muscle tissue.
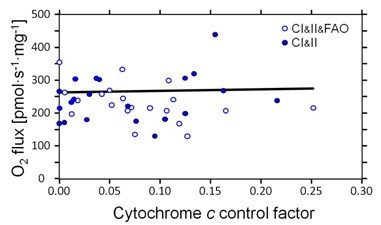
We aimed at establishing a reference method for the application of a cytochrome c threshold as exclusion criterion in mitochondrial OXPHOS analyses. Our study involved Alaskan sled dogs (N=6) studied 72 to 120 h after finishing a competitive 1,000 mile race in nine days. Permeabilized fibers (wet weight per chamber of 0.81-1.28 mg ± 0.12 SD) were prepared from needle biopsies and immediately studied by high-resolution respirometry [4] using 12 chambers in parallel (Oroboros Oxygraph-2k). Compared to human skeletal muscle fibers, the canine samples were more trexturally supple and sticky, requiring delicate fiber separation under light microscope, and disintegrating to various degrees during substrate-uncoupler-inhibitor titration (SUIT) protocols. This was reflected in variable and sometimes extremely high cytochrome c effects. However, there was no loss of CI- or CI&II-linked OXPHOS and ET capacity with increasing FCFc (Figure 1). Apparently, the damage caused by mt-preparation even in cases with FCFc up to 0.25 could be rescued by addition of 10 µM c and thus restore capacities comparable with samples of negligible FCFc. In contrast, multiple defects associated with increasing FCFc in human muscle fibers cannot be compensated fully by addition of cytochrome c [2,5]. Cytochrome c was applied early in the two SUIT protocols, in the CI-linked or CI&FAO-linked OXPHOS state. This allowed consistent analysis of subsequent respiratory states which were all supported by the externally added cytochrome c (Figure 1).
OXPHOS and ET capacities with FAO- and CI&II-linked substrates were higher than in muscle from competitive horses and humans [5,6]. The present approach (Figure 1) allows evaluation of the FCFc threshold as a potential exclusion criterion in healthy controls.
• O2k-Network Lab: AT Innsbruck Oroboros, CA Vancouver Boushel RC, US OK Stillwater Davis MS, US CO Fort Collins Miller BF, US CO Fort Collins Hamilton K, US OK Oklahoma City Miller BF
Affiliation
1-Oroboros Instruments, Innsbruck, Austria; 2-The Swedish School Sports Health Sc, Lindigovagen, Sweden; 3-College Health Human Sc, Colorado State Univ., Fort Collins, CO, US; 4-Land O’Lakes Purina Feed, St Louis, MO, US, 5Comparative Exercise Physiol Lab, Center Veterinary Health Sc, Oklahoma State Univ, Stillwater, OK, US; 5D Swarovski Research Lab, Dep Visceral Transplant Thoracic Surgery, Medical Univ Innsbruck, Austria – [email protected]
Figures
Figure 1. Independence of O2 flux (ET capacity in the presence of cytochrome c) of the cytochrome c control factor,
FCFc = (JCHOc-JCHO)/JCHOc
ET capacity was 238±64 pmol∙s-1∙mg-1 Ww independent of the CHO substrate combination supporting CI&II-linked electron flow in the presence or absence of 0.2 mM octanoyl carnitine (FAO).
References and acknowledgements
Supported by K-Regio project MitoCom.
- Gnaiger E (2014) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 4th ed. Mitochondr Physiol Network 19.12. Oroboros MiPNet Publications, Innsbruck: 72 pp.
- Rasmussen HN, Rasmussen UF (1997) Small scale preparation of skeletal muscle mitochondria, criteria of integrity, and assays with reference to tissue function. Mol Cell Biochem 174: 55-60.
- Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Mark W, Steurer W, Saks V, Usson Y, Margreiter R, Gnaiger E (2004) Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am J Physiol Heart Circ Physiol 286: H1633–41.
- Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25-58.
- Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41: 1837-45.
- Votion DM, Gnaiger E, Lemieux H, Mouithys-Mickalad A, Serteyn D (2012) Physical fitness and mitochondrial respiratory capacity in horse skeletal muscle. PLoS One 7: e34890.
Labels: MiParea: Respiration
Organism: Dog
Tissue;cell: Skeletal muscle
Preparation: Permeabilized tissue
Regulation: Cyt c Coupling state: LEAK, ROUTINE, OXPHOS, ET Pathway: N, NS HRR: Oxygraph-2k Event: C2, Poster MiP2014