Kane 2016 Abstract MitoFit Science Camp 2016
| Effects of inhibiting myosin-ATPase on mitochondrial respiratory capacity in permeabilized skeletal muscle. |
Link:
Kane DA, Brebner K, Perry CGR, Neufer PD (2016)
Event: MitoFit Science Camp 2016 Kuehtai AT
Unlike isolated mitochondria, respiratory oxygen fluxes (JO2) in permeabilized myofibers exhibit a dependence on oxygen level, decreasing non-linearly at or below air-saturation in standard assay media [1]. Anecdotally, permeabilized myofibers can degrade unpredictably during respirometric assay, precluding normalization of respiration to sample dry weight post-experiment. Pharmacologically inhibiting contraction has been suggested as a way to both prevent tissue degradation, as well as lessen the dependence of permeabilized myofibers on oxygen for reliable JO2 [2]. A potential source of inconsistency in respiration with permeabilized fibers may involve small differences in sample handling prior to assay (e.g., conditions under, and extent to which fibers are mechanically separated, precise wash temperatures, pre-assay weighing), which could influence the contractile state of the fibers. An obvious way around any potential pre-assay inconsistency might, therefore, be to introduce contraction inhibitor early in the preparation of the sample.
The purpose of these experiments was to determine whether JO2 in myofibers treated with myosin II ATPase inhibitor blebbistatin (bleb) during the entire sample preparation exhibit different JO2 when assayed in standard MiR06 sucrose respiration media.
Very large (28.98 ± 2.10 mg; to speed oxygen consumption), red gastrocnemius samples from male Wistar rats (N = 3) were prepared (i.e., saponin-permeabilized 30 min, washed 3 h) with or without bleb, and assessed for JO2 with or without bleb using high-resolution respirometry in otherwise standard conditions (e.g., MiR06 sucrose assay buffer), and at various oxygen concentrations. JO2 was expressed as pmol/s/mg wet weight. Data were analyzed using two-way repeated measures ANOVA, with Bonferroni post-hoc test (α = 0.05).
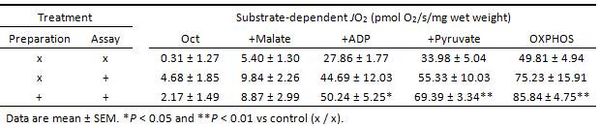
No differences in JO2 were observed among substrate conditions prior to addition of ADP. However, ADP-stimulated (5 mM) JO2 with either octanoyl-L-carnitine (0.4 mM) +malate (2 mM) or +pyruvate (5 mM) was significantly greater (P < 0.05 and 0.01, respectively) when samples were both prepared and assayed in bleb under hyperoxic conditions compared to control (control = no bleb during preparation or assay). These differences in JO2 between bleb and control persisted upon re-hyperoxygenation (OXPHOS; P < 0.01). Regardless of treatment, JO2 in permeabilized fibers exhibited classic dependence on oxygen, decreasing non-linearly at and below about 100-200 µM O2 (Figure 1).
These results demonstrate (1) greater ADP-stimulated JO2 in permeabilized myofibers with bleb when contraction is inhibited during both preparation and assay; and (2) dependence of JO2 in permeabilized myofibers to O2 level in sucrose media, regardless of bleb.
• O2k-Network Lab: CA Antigonish Kane DA, CA Guelph Holloway GP, CA Toronto Perry CG, US NC Greenville Neufer PD
Labels: MiParea: Respiration
Stress:Oxidative stress;RONS Organism: Rat Tissue;cell: Skeletal muscle Preparation: Permeabilized tissue
Regulation: Inhibitor, Oxygen kinetics Coupling state: OXPHOS Pathway: F, N HRR: Oxygraph-2k Event: C1 MitoFit Science Camp 2016
Affiliations
1-Dept Human Kinetics, St. Francis Xavier Univ, Angonish; 2-School Kinesiol Health Sc, Norman Bethune College, Toronto; CA; 3-Vanderbilt Univ, Nashville, TN; 4-East Carolina Univ, Greenville, NC; US. - [email protected]
Figures
Figure 1. Respiratory O2 flux (JO2) in large, saponin-permeabilized red gastrocnemius samples from male Wistar rats (N = 3) prepared (i.e., saponin-permeabilized 30 min, washed 3 h) with (+) or without bleb (x). Samples were also assessed for JO2 with (+) or without bleb (x) using high-resolution respirometry in otherwise standard conditions (e.g., MiR06 sucrose assay buffer), and at various O2 concentrations (0 – 600 µM). Values for JO2 are expressed as pmol/s/mg wet weight. Protocol: octanoyl-L-carnitine (Oct; 0.4 mM), malate (+Malate; 2 mM), pyruvate (+Pyruvate; 5 mM). OXPHOS = greatest recorded JO2 following addition of ADP and re-hyperoxygenation.
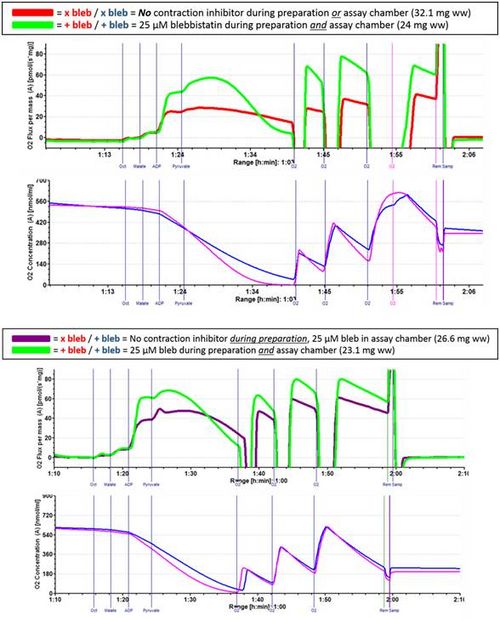
Figure 2. Representative respirometric traces of large, saponin-permeabilized red gastrocnemius samples from male Wistar rats (N = 3) prepared (i.e., saponin-permeabilized 30 min, washed 3 h) with (+) or without bleb (x). Samples were also assessed for JO2 with (+) or without bleb (x) using high-resolution respirometry in otherwise standard conditions (e.g., MiR06 sucrose assay buffer), and at various O2 concentrations (0 – 600 µM). Protocol: octanoyl-L-carnitine (Oct; 0.4 mM), malate (+Malate; 2 mM), pyruvate (+Pyruvate; 5 mM). Data illustrate impact of bleb and dependence of JO2 on [O2] in permeabilized fibers.
References
- Scandurra FM, Gnaiger E (2010) Cell respiration under hypoxia: facts and artefacts in mitochondrial oxygen kinetics. Adv Exp Med Biol 662:7-25.
- Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD (2011) Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J 437:215-22.