Ekbal 2013 Chest
| Ekbal NJ, Dyson A, Black C, Singer M (2013) Monitoring tissue perfusion, oxygenation, and metabolism in critically ill patients. Chest 143:1799-1808. https://doi.org/10.1378/chest.12-1849 |
Ekbal NJ, Dyson A, Black C, Singer M (2013) Chest
Abstract: Alterations in oxygen transport and use are integral to the development of multiple organ failure; therefore, the ultimate goal of resuscitation is to restore effective tissue oxygenation and cellular metabolism. Hemodynamic monitoring is the cornerstone of management to promptly identify and appropriately manage (impending) organ dysfunction. Prospective randomized trials have confirmed outcome benefit when preemptive or early treatment is directed toward maintaining or restoring adequate tissue perfusion. However, treatment end points remain controversial, in large part because of current difficulties in determining what constitutes "optimal." Information gained from global whole-body monitoring may not detect regional organ perfusion abnormalities until they are well advanced. Conversely, the ideal "canary" organ that is readily accessible for monitoring, yet offers an early and sensitive indicator of tissue "unwellness," remains to be firmly identified. This review describes techniques available for real-time monitoring of tissue perfusion and metabolism and highlights novel developments that may complement or even supersede current tools.
• Bioblast editor: Gnaiger E
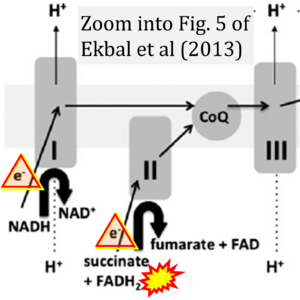
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase