Chance 1955 J Biol Chem-III
| Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation: III. The steady state. J Biol Chem 217:409-27. |
Chance B, Williams GR (1955) J Biol Chem
Abstract: The initiation of oxidative phosphorylation by the addition of ADP to a suspension of liver mitochondria causes changes in the steady state of the components of its respiratory chain. Spectrophotometric methods are used to measure the changes in five components. With β-hydroxybutyrate as a substrate for rat liver mitochondria there is a graded series of changes from a 4 per cent reduction of cytochrome a, an 8 per cent oxidation of cytochrome c, a 19 per cent oxidation of cytochrome b, and a 20 per cent oxidation of flavoprotein to a 53 per cent oxidation of DPNH. Thus we find the whole respiratory chain to be affected by ADP and do not observe any single component of the chain to be responsible for oxidative phosphorylation. Three pairs of respiratory enzymes have been found to be involved in an inhibition phenomenon brought on by the exhaustion of added ADP. DPNH is found to exist in an inhibited form in mitochondria lacking ADP, and, by analogy, it is probable that the components of the other two pairs of respiratory enzymes that are similarly inhibited are the reduced forms of cytochromes c and b. • Keywords: Respiratory enzymes, Respiratory chain, Steady state, OXPHOS, Liver mitochondria • Bioblast editor: Gnaiger E
Selected quotes
- In a complex enzymatic sequence, the site of action of substrates and inhibitors is clearly marked by the way they affect the steady state concentrations of the components of the system. Antimycin A, for example, inhibits respiration in the succinic oxidase system and at the same time increases the steady state reduction of cytochrome b and decreases that of cytochromes c, a, and aa3. In the oxidative phosphorylation system of liver mitochondria, phosphate and phosphate acceptors cause a considerable activation of respiration and may do so by a reversal of inhibitory reactions along the respiratory chain. Thus measurements of changes in the steady state of the members of the respiratory chain upon initiation and cessation of oxidative phosphorylation of ADP may identify sites in the chain where the phosphorylation reactions occur.
- In measurements of small optical density changes in the mitochondrial suspension by means of the double beam technique the mitochondria are diluted 5- to lo-fold (to 4 to 8 mg of protein per mL) in the isotonic reaction medium ..
- Thus intramitochondrial DPN can be further reduced by the exogenous substrate, and, as later data show, this reduction is very nearly as great as that caused by anaerobiosis.
- .. we have plotted the extent of oxidation as a function of added ADP concentration, and, as Fig. 2, B shows, 56 µM ADP give half-maximal effect.
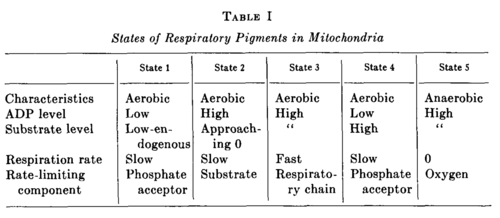
- State 1: The initial optical density of the suspension corresponds to State 1, and, as Fig. 1 shows, DPN is not fully reduced by the endogenous substrate.
- State 2: Fig. 3 demonstrates that addition of ADP to State 1 causes an oxidation of DPNH and cytochrome b, and, according to Fig. 6, the oxidation goes very nearly to completion in State 2. This oxidation of DPNH is caused by a direct effect of ADP upon the respiratory chain and by the exhaustion of endogenous substrate utilized in the oxidative phosphorylation of ADP.
- State 3: State 3, which is reached in Fig. 3 by the addition of β-hydroxybutyrate and corresponds to a considerable reduction of DPN and cytochrome b, is the state of oxidative phosphorylation and terminates when the added ADP has been phosphorylated.
- State 4: Then State 4 is reached without further addition of reactants. That State 4 corresponds to the exhaustion of ADP is verified by the coincident decrease in respiration measured with the platinum microelectrode (cf. Fig. 1). The transition from State 3 to 4 corresponds to a further reduction of DPN and cytochrome b.
- State 5: In the course of a minute or so the small amount of dissolved oxygen remaining in the cuvette is utilized, and the mitochondrial suspension becomes anaerobic to give State 5.
- Since our interest is focused upon the specific effect of ADP upon the steady state, the transition from State 3 to 4 is reversed by a second addition of ADP to State 4 to give State 3 again. This is the transition that is specifically caused by the initiation of oxidative phosphorylation and is uncomplicated by the exhaustion of endogenous substrate as is the case when ADP is added to State 1.
- State 5 may be obtained by antimycin A treatment or by anaerobiosis, provided measurements are made in the visible region of the spectrum.
- At wave-lengths appropriate for the measurement of flavoprotein (Equation 1) we observe that ADP additions cause a downward deflection of the trace (corresponding to an increase of optical density at 465 mp), which indicates an oxidation of flavoprotein on reaching State 2. The remaining stages follow the pattern for cytochrome b, and State 4 does not give complete reduction. The level of reduction on which the percentage changes in the steady state are calculated is based upon the effect of antimycin A (in this case the effects of antimycin A and anaerobiosis differ (10)). The values of flavoprotein reduction are 31 and 17 percent for States 4 and 3, respectively.
- Cytochrome c shows a pattern of spectroscopic changes that is also similar to cytochrome b, and in this case, the level of reduction used for calculation of percentage changes is that caused by anaerobiosis: States 4 and 3 correspond to 11 and 5 percent reduction of cytochrome c.
- The pattern of the ADP effect is a slight reduction of the terminal oxidase and an increasing degree of oxidation of the components along the respiratory chain until the lowest member is affected to an extent of about 50 percent. The pattern of the substrate effect is uniformly one of reduction throughout the chain.
- Succinate gives greater reduction for States 3 and 4 than β-hydroxybutyrate, especially in the case of flavoprotein, but with succinate the transition from States 2 to 3 is very slow for all components, as if a more reactive substance were slowly being formed from the added succinate.
- The general rule for steady state changes is a consistent gradation of the effect along the respiratory chain from an oxidation of the dehydrogenase to a reduction of the oxidase system. The point of crossover from oxidation to reduction is usually between cytochromes c and a but may occur between cytochromes c and b or between flavoprotein and DPNH, depending upon the relative activities of the components of the respiratory chain or upon the presence of different substrates or inhibitors.
- .. it is justifiable to take State 2 as very nearly completely oxidized.
- For cytochromes a and c, the anaerobic level can be taken as 100 percent reduced, because hydrosulfite addition to the anaerobic mitochondria produces no further reduction.
- In order to evaluate the amount of the flavoprotein that may be involved in DPNH oxidation, we have observed a further reduction of flavoprotein upon addition of antimycin A to mitochondria in State 4 and find this to be only two-thirds of the amount reduced in anaerobiosis in most guinea pig and rat liver preparations.
Definition of States 1 to 5
Made history
Cited by
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1.
Labels: MiParea: Respiration
Organism: Rat, Guinea pig
Tissue;cell: Liver
Preparation: Isolated mitochondria
Coupling state: LEAK, OXPHOS
Pathway: N, S
Made history, LEAK respiration, Respiratory states, Steady state, BEC 2020.1, BEC 2020.2, MitoFit 2022 NADH, Ambiguity crisis