Brzezinski 2021 Chem Rev
| Brzezinski P, Moe A, Ädelroth P (2021) Structure and mechanism of respiratory III-IV supercomplexes in bioenergetic membranes. Chem Rev 121:9644-73. https://doi.org/10.1021/acs.chemrev.1c00140 |
Brzezinski P, Moe A, Aedelroth P (2021) Chem Rev
Abstract: In the final steps of energy conservation in aerobic organisms, free energy from electron transfer through the respiratory chain is transduced into a proton electrochemical gradient across a membrane. In mitochondria and many bacteria, reduction of the dioxygen electron acceptor is catalyzed by cytochrome c oxidase (Complex IV), which receives electrons from cytochrome bc1 (Complex III), via membrane-bound or water-soluble cytochrome c. These complexes function independently, but in many organisms they associate to form supercomplexes. Here, we review the structural features and the functional significance of the nonobligate III2IV1/2 Saccharomyces cerevisiae mitochondrial supercomplex as well as the obligate III2IV2 supercomplex from actinobacteria. The analysis is centered around the Q-cycle of Complex III, proton uptake by CytcO, as well as mechanistic and structural solutions to the electronic link between Complexes III and IV.
• Bioblast editor: Gnaiger E
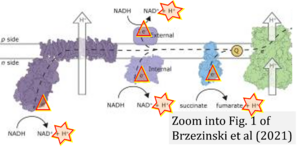
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Organism: Saccharomyces cerevisiae, Eubacteria
Enzyme: Complex I, Complex II;succinate dehydrogenase, Complex III, Complex IV;cytochrome c oxidase, Supercomplex