BEC tutorial-Living Communications: pmF to pmP
From Bioblast
- Mitochondrial membrane potential and Peter Mitchell’s protonmotive force pmF: elements of the science of bioenergetics
Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. https://doi.org/10.26124/bec:2020-0002 - Chapter 8: Protonmotive pressure and respiratory control
- The mitochondrial membrane potential is an element of the science of bioenergetics, linked to the control of respiratory flux and related mitochondrial functions. A PubMed search on ‘mitochondrial membrane potential’ yields nearly 40 000 results and 3442 for 2021 (search 2022-07-04), with a linear increase during the past 20 years. Chapter 8 on ‘Protonmotive pressure and respiratory control’ of Mitochondrial Pathways (Gnaiger 2020) introduces a novel perspective on Peter Mitchell’s protonmotive force, which incorporates the mitochondrial membrane potential. If you find the reading is tough, you are not alone. Join this BEC tutorial-Living Communications for a fundamental introduction into the relevant concepts of physical chemistry, which differ from misleading chapters in bioenergetics textbooks. A retreat with plenty of informal discussions and group interactions takes you on a journey to visit chemical potential differences versus potential gradients, Gibbs energy versus Gibbs force, quantities of capacity versus intensity, protonmotive force and motive units, flows and forces, and finally protonmotive pressure. This will introduce students (and teachers) to a new understanding of mitochondrial membrane potential and the protonmotive force, connecting the ideal gas equation, osmotic pressure, the Boltzmann constant and gas constant with Fick’s and Einstein’s diffusion equation.
BEC tutorials-Living Communications: pmF to pmP
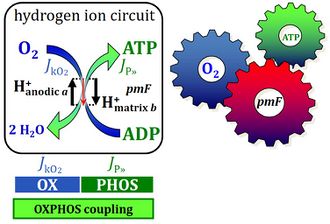
pmF: a unifying theory of biology - from metabolism to physical chemistry
- Peter Mitchell's concept of the protonmotive force pmF is one of the grand unifying theories of biology, on par with Charles Darwin's theory of evolution, Gregor Mendel's rules of inheritance and classical genetics, and the structure of DNA resolved by Francis Crick, James Watson, and Rosalind Franklin. The pmF combines the disciplines of biochemistry (metabolism), cell biology (cellular ultrastructure), physiology (energy transformation), thermodynamics (chemical potential, Gibbs energy), and physical chemistry (diffusion, electrochemistry).
- This BEC tutorial links different disciplines and describes different processes (transformations) by the same principles and relations of isomorphic quantities:
- metabolic reactions and translocation — scalar and vectorial
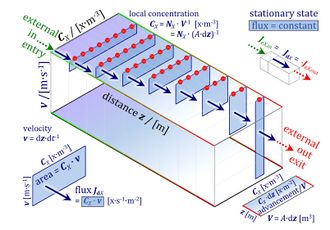
- diffusion — from Fick's law to Einstein's diffusion equation
- electrochemical potentials — compartmental differences versus gradients — and motive forces of physics and thermodynamics - from the Boltzmann constant and gas constant to the electromotive constant
- osmotic pressure — from the gas law to protonmotive pressure
- Remember ZEN, zeNA — Section 8.2.8 - ∞2∞
- RM Pirsig (1974) Zen and the art of motorcycle maintenance. An inquiry into values. William Morrow & Company:418 pp.
- Thermodynamics (motorcycle maintenance) may be dull and tedious drudgery (without curiosity beyond “≡”) or a valuable and exciting art (if you seek for “=” ZEN). Transformation of dumb, dry and frigid equations into eloquent formulae radiating meaning and sparkling knowledge depends on motivation, skill and persistence (zeNA).
- » Compare numerical equivalence (symbol ≡) and physicochemical equality (symbol =).
Why?
- Why are mitochondria small? Why is LEAK respiration a non-linear (non-ohmic) function of the mitochondrial membrane potential difference ΔΨp+?
- Why is the mitochondrial membrane potential difference ΔΨp+ — the chemical part of the pmF — not a force of physics? Similarly, the protonmotive force is not a force of physics. Why 'isomorphic' forces?
- Why can we start a chemical reaction (in a homogenous system) or compartmental diffusion (in a discontinuous system) at an infinitely large force - without the system exploding?
Consider some fundamental quantities
- Bioblast links: Force and membrane potential - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Fundamental relationships
- mt-Membrane potential and protonmotive force
- O2k-Potentiometry
- » O2k-Catalogue: O2k-TPP+ ISE-Module
- » O2k-Manual: MiPNet15.03 O2k-MultiSensor-ISE
- » TPP - O2k-Procedures: Tetraphenylphosphonium
- » Specifications: MiPNet15.08 TPP electrode
- » Poster
- » Unspecific binding of TPP+
- » TPP+ inhibitory effect
- O2k-Potentiometry
- O2k-Fluorometry
- » O2k-Catalogue: O2k-FluoRespirometer
- » O2k-Manual: MiPNet22.11 O2k-FluoRespirometer manual
- » Safranin - O2k-Procedures: MiPNet20.13 Safranin mt-membranepotential / Safranin
- » TMRM - O2k-Procedures: TMRM
- O2k-Fluorometry
- O2k-Publications
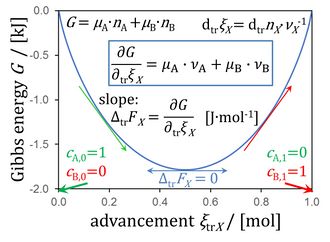
- Among the key isomorphic quantities are:
- advancement and stoichiometry as the determinants of transformation flows
- motive entity - this is what flows
- motive units for count, amount, and charge
- chemical and electric partial forces of the pmF
- Important distinctions:
- systems: closed, compartmental, open
- transformations: vectoral (along continuous gradients), vectorial (across discontinuous boundaries between compartments), scalar (within systems, without spatial direction)
- Gibbs energy (exergy), chemical potential, and metabolic force (Gibbs force)
- potential gradients versus potential differences
- protons p+ and hydrogen ions H+
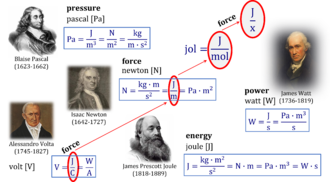
- (chemiosmotic) pressure versus (protonmotive) force
Gibberish
- Forget all gibberish that you have learned — if not forgotten already — on textbook thermodynamics. If you are surprised by this suggestion, take a look at specific examples from
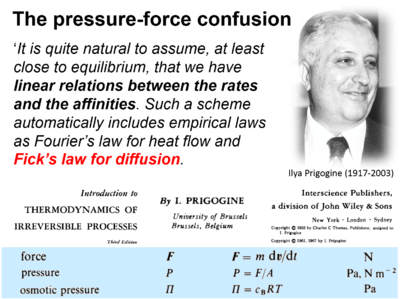
Force or pressure? - The linear flux-pressure law
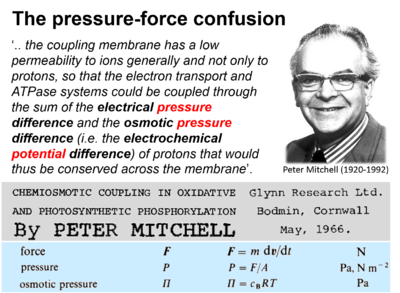
- "For many decades the pressure-force confusion has blinded the most brilliant minds, reinforcing the expectation that Ohm’s linear flux-force law should apply to the hydrogen ion circuit and protonmotive force. .. Physicochemical principles explain the highly non-linear flux-force relation in the dependence of LEAK respiration on the pmF. The explanation is based on an extension of Fick’s law of diffusion and Einstein’s diffusion equation, representing protonmotive pressure ― isomorphic with mechanical pressure, hydrodynamic pressure, gas pressure, and osmotic pressure ― which collectively follow the generalized linear flux-pressure law."
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- » pressure = force × free activity
Recommended reading
- Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. https://doi.org/10.1016/j.bbabio.2011.09.018
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. https://doi.org/10.26124/bec:2020-0002 - Chapter 8
- Gnaiger E (2021) The elementary unit — canonical reviewer's comments on: Bureau International des Poids et Mesures (2019) The International System of Units (SI) 9th ed. https://doi.org/10.26124/mitofit:200004.v2