Andrieux 2021 Int J Mol Sci
| Andrieux P, Chevillard C, Cunha-Neto E, Nunes JPS (2021) Mitochondria as a cellular hub in infection and inflammation. Int J Mol Sci 22:11338. https://doi.org/10.3390/ijms222111338 |
Andrieux P, Chevillard C, Cunha-Neto E, Nunes JPS (2021) Int J Mol Sci
Abstract: Mitochondria are the energy center of the cell. They are found in the cell cytoplasm as dynamic networks where they adapt energy production based on the cell's needs. They are also at the center of the proinflammatory response and have essential roles in the response against pathogenic infections. Mitochondria are a major site for production of Reactive Oxygen Species (ROS; or free radicals), which are essential to fight infection. However, excessive and uncontrolled production can become deleterious to the cell, leading to mitochondrial and tissue damage. Pathogens exploit the role of mitochondria during infection by affecting the oxidative phosphorylation mechanism (OXPHOS), mitochondrial network and disrupting the communication between the nucleus and the mitochondria. The role of mitochondria in these biological processes makes these organelle good targets for the development of therapeutic strategies. In this review, we presented a summary of the endosymbiotic origin of mitochondria and their involvement in the pathogen response, as well as the potential promising mitochondrial targets for the fight against infectious diseases and chronic inflammatory diseases.
• Bioblast editor: Gnaiger E
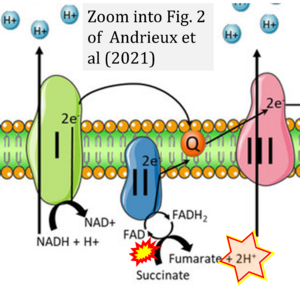
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
- Fig. 2 of Andrieux et al (2021) indicates oxidation of FADH2 to FAD linked to oxidation of succinate to fumarate + 2H+. Apart from the fact that (1) FAD/FADH2 are covalently bound within CII (succinate is the substrate of CII comparable to NADH + H+ as the substrates for CI) and (2) formation of 2H+ during oxidation of succinate to fumarate lacks justification, the redox reaction requires reduction of FAD to FADH2 to be linked to oxidation of succinate to fumarate. Perhaps open instead of anonymous peer review might encourage a scientific team spirit to avoid publishing such a confusing figure. Additionally, the editors of pro-profit journals should take an appropriate responsibility to improve their contribution to quality control. The trust of the public in science and the motivation of students to read scientific literature are at stake.
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«