Granata 2015 Nutr Metab (Lond)
| Granata S, Dalla Gassa A, Tomei P, Lupo A, Zaza G (2015) Mitochondria: a new therapeutic target in chronic kidney disease. Nutr Metab (Lond) 12:49. https://doi.org/10.1186/s12986-015-0044-z |
Granata S, Dalla Gassa A, Tomei P, Lupo A, Zaza G (2015) Nutr Metab (Lond)
Abstract: Cellular metabolic changes during chronic kidney disease (CKD) may induce higher production of oxygen radicals that play a significant role in the progression of renal damage and in the onset of important comorbidities. This condition seems to be in part related to dysfunctional mitochondria that cause an increased electron "leakage" from the respiratory chain during oxidative phosphorylation with a consequent generation of reactive oxygen species (ROS). ROS are highly active molecules that may oxidize proteins, lipids and nucleic acids with a consequent damage of cells and tissues. To mitigate this mitochondria-related functional impairment, a variety of agents (including endogenous and food derived antioxidants, natural plants extracts, mitochondria-targeted molecules) combined with conventional therapies could be employed. However, although the anti-oxidant properties of these substances are well known, their use in clinical practice has been only partially investigated. Additionally, for their correct utilization is extremely important to understand their effects, to identify the correct target of intervention and to minimize adverse effects. Therefore, in this manuscript, we reviewed the characteristics of the available mitochondria-targeted anti-oxidant compounds that could be employed routinely in our nephrology, internal medicine and renal transplant centers. Nevertheless, large clinical trials are needed to provide more definitive information about their use and to assess their overall efficacy or toxicity.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
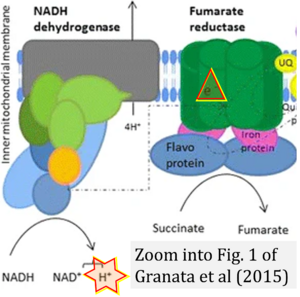
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.